不对称 Cu (II) 席夫碱配合物的结构、光谱和生物特性:合成、DNA/BSA 相互作用以及抗菌潜力的实验和计算见解
IF 2.6
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
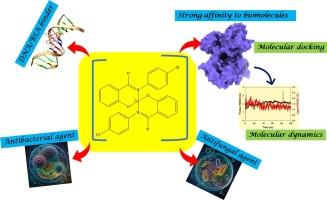
利用傅立叶变换红外光谱和紫外可见分光光度法合成并表征了铜 (II) 与不对称双齿席夫碱配体 (E)-2-(((4-chlorophenyl) imino) methyl) 苯酚的配合物。对铜(II)络合物和游离配体进行了光致发光研究。XRD 分析表明,铜 (II) 复合物为单斜晶系结晶,空间群为 P21/c。利用 B3LYP/6-31G+(d,p) 和 Lanl2dz 基集进行的 DFT 计算优化了络合物结构,QTAIM 和 NBO 分析证实了络合物的形成。铜 (II) 复合物的能隙被确定为 3.701 eV(α态)和 2.764 eV(β态)。对优化结构的 MEP 分析表明,氮原子周围存在疏水环境,分子具有整体疏水性。氯原子被确定为潜在的亲核位点。评估了希夫碱铜 (II) 复合物对某些致病细菌(枯草杆菌、大肠杆菌、肺炎双球菌、大肠杆菌)和真菌白色念珠菌的抗菌效果。其中,在 2 毫克/毫升和 2.5 毫克/毫升等较高浓度下,白念珠菌与复合物的结合区较大(11-15.5 毫米)。分子对接研究表明,复合物与目标蛋白质之间具有很强的结合亲和力,模拟过程中获得的均方根偏差、均方根波动、回旋半径和氢键值进一步证实了这一点。计算得出的 BSA 与铜(II)复合物之间的结合自由能为 -8.7 kcal/mol,表明存在显著的相互作用。然而,在复合物和 DNA 之间只观察到微弱的范德华相互作用。毒性研究表明,铜 (II) 复合物对眼部和皮肤组织的刺激作用很小,致死剂量也很低。相比之下,磷酸三甲苯酯对眼部有中度到严重的刺激作用,对皮肤有轻微的刺激作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Structural, spectroscopic, and biological properties of an asymmetric Cu (II) Schiff-base complex: Synthesis, DNA/BSA interactions, and antimicrobial potential with experimental and computational insights
A copper (II) complex with the asymmetric bidentate Schiff-base ligand (E)-2-(((4-chlorophenyl) imino) methyl) phenol was synthesized and characterized using FTIR and UV–VIS spectroscopy. Photoluminescence studies were conducted on both the copper (II) complex and the free ligand. XRD analysis revealed the copper (II) complex crystallizes in the monoclinic system, space group P21/c. The complex structure was optimized using DFT calculations with B3LYP/6-31G+(d,p) and Lanl2dz basis sets, with QTAIM and NBO analyses confirming complex formation. The energy gap of the copper (II) complex was determined to be 3.701 eV (alpha state) and 2.764 eV (beta state). MEP analysis of the optimized structure indicated a hydrophobic environment around the nitrogen atom, with the molecule exhibiting overall hydrophobicity. The chlorine atoms were identified as potential nucleophilic sites. The antimicrobial efficacy of a Schiff base copper (II) complex was evaluated against selected pathogenic bacteria (B. subtilis, B. megaterum, K. pneumoniae, E. coli) and the fungus Candida albicans. Among these C. albicans for complex showed larger zones (11–15.5 mm) at higher concentrations such as 2 and 2.5 mg/mL. Molecular docking studies suggested strong binding affinity between the complex and the target protein, this was further supported by obtained root means square deviations, root means square fluctuations, radius of gyration and hydrogen bond values during simulations. The binding free energy between BSA and the copper (II) complex was calculated as −8.7 kcal/mol, indicating significant interaction. However, only weak van der Waals interactions were observed between the complex and DNA. Toxicity studies revealed the copper (II) complex had minimal irritant effects on ocular and cutaneous tissues, with a low lethal dose. In comparison, Tricresyl phosphate showed moderate to severe ocular irritancy and modest cutaneous irritancy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Polyhedron
化学-晶体学
CiteScore
4.90
自引率
7.70%
发文量
515
审稿时长
2 months
期刊介绍:
Polyhedron publishes original, fundamental, experimental and theoretical work of the highest quality in all the major areas of inorganic chemistry. This includes synthetic chemistry, coordination chemistry, organometallic chemistry, bioinorganic chemistry, and solid-state and materials chemistry.
Papers should be significant pieces of work, and all new compounds must be appropriately characterized. The inclusion of single-crystal X-ray structural data is strongly encouraged, but papers reporting only the X-ray structure determination of a single compound will usually not be considered. Papers on solid-state or materials chemistry will be expected to have a significant molecular chemistry component (such as the synthesis and characterization of the molecular precursors and/or a systematic study of the use of different precursors or reaction conditions) or demonstrate a cutting-edge application (for example inorganic materials for energy applications). Papers dealing only with stability constants are not considered.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


