新型米诺环素/锰复合物的光谱和化学特性及其对大肠杆菌(ATCC 8739)、枯草芽孢杆菌(ATCC6633)、金黄色葡萄球菌(ATCC 6538)和肺炎克雷伯菌(ATCC 13883)的高效抗氧化活性和抗菌效果的体外评价
IF 2.4
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
背景和目的米诺环素(Mino)是一种广谱抗菌药,能迅速被完全吸收。米诺被认为是一种独特的四环素衍生物。近来,市售抗菌剂的数量有所下降,跟不上治疗对多种药物产生耐药性的病原体的挑战数量。材料与方法通过化学方法合成了米诺/锰(Mino/Mn)复合物新配方,并利用元素分析、多种光谱方法(红外(IR)、紫外(UV)、X 射线衍射(XRD))、磁感应强度、扫描电子显微镜(SEM)和透射电子显微镜(TEM)对其进行了全面表征。摩尔电导值证实了米诺/锰新型复合物的非电解性质。新型 Mino/Mn 复合物使用 ORAC 法进行了测试,并评估了其对以下菌株的抗菌活性:结果光谱数据显示,Mino 通过酮基 (CO) 和 (-OH) 羟基的氧原子与 Mn(II) 螯合。磁矩值证实了络合物的八面体构型。通过扫描电子显微镜观察新型络合物 Mino/Mn 的表面形态,证实该新型络合物呈小矩形突起。TEM 显示锰(II)螯合物形成了黑点,粒径在 9 至 23 纳米之间。目前的研究结果证实,米诺/锰络合物在极低浓度下对上述四种菌株具有很高的抗菌活性:大肠杆菌为 0.625 毫克/毫升,枯草杆菌为 0.009 毫克/毫升,金黄色葡萄球菌为 0.625 毫克/毫升。米诺/锰复合物通过捕捉和清除滥用抗生素产生的自由基,表现出了强大的抗氧化活性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
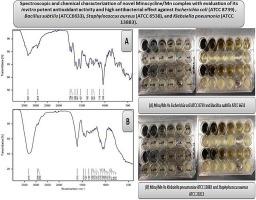
Spectroscopic and chemical characterization of the novel Minocycline/Mn complex with evaluation of its invitro potent antioxidant activity and high antibacterial effect against Escherichia coli (ATCC 8739), Bacillus subtilis (ATCC6633), Staphylococcus aureus (ATCC 6538), and Klebsiella pneumonia (ATCC 13883)
Background and objective
Minocycline (Mino) is a broad-spectrum antimicrobial that is quickly and fully absorbed. Mino is considered a unique tetracycline derivative. Recently, the number of commercially available antibacterial agents has declined, failing to keep pace with the number of challenges of treating pathogens resistant to multiple drugs. Therefore, it is necessary to develop new classes of antibiotics with different modes of action.
Material and methods
The Mino/manganese (Mino/Mn) complex novel formula was chemically synthesized and fully characterized by using elemental analysis, a lot of spectroscopic methods (infrared (IR), ultraviolet (UV), X-ray diffraction (XRD)), magnetic susceptibility, scanning electron microscopy SEM, and transmission electron microscopy (TEM). The molar conductance value confirmed the non-electrolytic nature of Mino/Mn novel complex. The novel Mino/Mn complex was tested using the ORAC assay and evaluated for its antibacterial activity against the following strains: Escherichia coli (ATCC 8739), Bacillus subtilis (ATCC 6633), Staphylococcus aureus (ATCC 6538), and Klebsiella pneumonia (ATCC 13883).
Results
Spectral data showed that Mino is chelated with Mn(II) via the oxygen atoms through the oxygen of ketonic group (C![]() O) and (-OH) hydroxyl groups. The magnetic moment value confirms the octahedral configuration of the complex. The surface morphology of the novel complex Mino/Mn that was observed via SEM confirmed that the novel complex appeared as small rectangular projections. TEM showed the formation of black spots for Mn(II) chelate with particle sizes in between 9 to 23 nm. The current findings confirmed the high antibacterial activity of the Mino/Mn complex against the four mentioned strains of bacteria at extremely low concentrations: 0.625 mg/ml for E. coli, 0.009 for B. subtilis, and 0.625 for S. aureus. The Mino/Mn complex exhibited potent antioxidant activity by capturing and scavenging free radicals resulting from antibiotic misuse.
O) and (-OH) hydroxyl groups. The magnetic moment value confirms the octahedral configuration of the complex. The surface morphology of the novel complex Mino/Mn that was observed via SEM confirmed that the novel complex appeared as small rectangular projections. TEM showed the formation of black spots for Mn(II) chelate with particle sizes in between 9 to 23 nm. The current findings confirmed the high antibacterial activity of the Mino/Mn complex against the four mentioned strains of bacteria at extremely low concentrations: 0.625 mg/ml for E. coli, 0.009 for B. subtilis, and 0.625 for S. aureus. The Mino/Mn complex exhibited potent antioxidant activity by capturing and scavenging free radicals resulting from antibiotic misuse.
Conclusion
Therefore, it can be concluded that the novel formula of the Mino/Mn complex is an effective antioxidant and antibacterial agent with expected high potentially significant biological effects.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Polyhedron
化学-晶体学
CiteScore
4.90
自引率
7.70%
发文量
515
审稿时长
2 months
期刊介绍:
Polyhedron publishes original, fundamental, experimental and theoretical work of the highest quality in all the major areas of inorganic chemistry. This includes synthetic chemistry, coordination chemistry, organometallic chemistry, bioinorganic chemistry, and solid-state and materials chemistry.
Papers should be significant pieces of work, and all new compounds must be appropriately characterized. The inclusion of single-crystal X-ray structural data is strongly encouraged, but papers reporting only the X-ray structure determination of a single compound will usually not be considered. Papers on solid-state or materials chemistry will be expected to have a significant molecular chemistry component (such as the synthesis and characterization of the molecular precursors and/or a systematic study of the use of different precursors or reaction conditions) or demonstrate a cutting-edge application (for example inorganic materials for energy applications). Papers dealing only with stability constants are not considered.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


