基于 3-羟基异烟酸的含 RECo 的金属有机框架:质子传导和磁性
IF 4
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
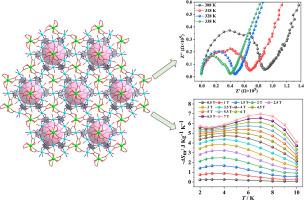
以稀土(RE)和过渡金属离子以及 3-羟基异烟酸(H2L)有机配体为基础,通过水热法合成了三种异金属金属有机框架,即 {RE2Co3L6(H2O)6}n(RE = Gd (1)、Dy (2)、Y(3))。单晶 X 射线衍射显示,1-3 号化合物具有同构和三维(3D)多孔结构。在化合物 1 中,Gd3+ 离子位于 C3 轴上,每两个螺旋桨状的 {GdCo3} 单元通过十二个 L2- 配体上下封盖到一个 {Gd6Co6} 环上,从而形成空穴。值得注意的是,通过热重分析(TGA)测定,这三种化合物具有较高的热稳定性,而将三种样品在水中浸泡三天前后的 PXRD 曲线的良好匹配也证明了它们具有良好的水稳定性。交流阻抗测量结果表明,化合物 1-3 与粉末样品的质子传导性明显依赖于温度和湿度,在 98% 相对湿度和 338 K 条件下,其值分别可达 3.38×10-5、1.91×10-5 和 2.23×10-5 S cm-1。此外,化合物 1-3 显示出反铁磁性行为,磁致效应(MCE)研究表明,在 7 K 和 7 T 条件下,化合物 1 的熵变 (-ΔSm) 为 7.1 J kg-1 K-1。本文章由计算机程序翻译,如有差异,请以英文原文为准。
RECo-containing metal-organic frameworks based on 3-hydroxyisonicotinic acid: Proton conduction and magnetism
Three heterometallic metal-organic frameworks, namely, {RE2Co3L6(H2O)6}n (RE = Gd (1), Dy (2), Y(3)), have been hydrothermally synthesized based on rare-earth (RE) and transition metal ions and the organic ligand of 3-hydroxyisonicotinic acid (H2L). Single-crystal X-ray diffraction reveals that compounds 1‒3 are isomorphic and features three-dimensional (3D) porous structure. In compound 1, the Gd3+ ion is located in the C3 axis, and every two propeller-like {GdCo3} units are capped up and down to a {Gd6Co6} ring through twelve L2‒ ligands, giving rise to the formation of the cavities. Notably, three compounds display high thermal stability determined by thermogravimetric analysis (TGA), and good water stability proved by the well-matched of PXRD curves before and after soaked three samples in water for three days. AC impedance measurements exhibit the proton conductivities of compounds 1‒3 with powder samples are distinctly temperature and humidity dependent, and the values can reach 3.38×10‒5, 1.91×10‒5, and 2.23×10‒5 S cm‒1 at 98% RH and 338 K, respectively. Moreover, compounds 1‒3 display antiferromagnetic behaviours, and the magnetocaloric effect (MCE) study indicates that the entropy change (−ΔSm) of compound 1 is 7.1 J kg−1 K−1 at 7 K and 7 T. Compound 2 exhibits the slow magnetic relaxation behaviour.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


