氧化还原无辜的肼基甲酸酯官能化菲罗啉的合成、表征、单晶结构和抗霉菌及抗癌活性评估
IF 4
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
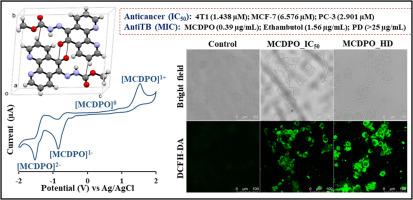
由于癌症和细菌细胞对已暴露的药物分子表现出抗药性,因此开发治疗癌症和细菌感染的生物活性候选药物一直是一项艰巨的挑战。肼基甲酸甲酯衍生菲罗啉化合物 6-[2-(甲氧羰基)偶氮-1-基]-1,10-菲罗啉-5-酮(MCDPO)是由 1,10-菲罗啉通过三步反应合成的。通过 HRMS、IR、1H NMR 和 13C NMR、紫外-可见光谱研究了 MCDPO 的光谱特征。单晶 XRD 研究证实了 MCDPO 的三维分子结构。MCDPO 在三linic 体系 P-1 空间群中结晶,单胞参数如下:a = 5.0347(2) Å,b = 10.3509(4) Å,c = 11.8816(4) Å,α = 84.431(3)° ,β = 84.172(3)° ,γ = 81.380(4)° ,V = 606.92(4) Å3 ,T = 133 K。MCDPO 含有芳香环、氢键供体和氢键受体基团,通过 C-H⋯O、CH⋯N、C-O⋯C、π-π 和 π⋯C 等非共价相互作用形成超分子分子排列。通过循环伏安法和基于紫外可见光谱的电化学方法研究了 MCDPO 的电化学氧化还原和电致变色特性。该化合物具有良好的抗癌活性,对 4T1、MCF-7 和 PC-3 癌细胞株的 IC50 值分别为 1.438、6.576 和 2.901 µM。对 4T1 细胞进行的流式细胞术研究表明,MCDPO 可通过诱导细胞凋亡和细胞周期停滞在 G0/G1 期来促进癌细胞死亡。此外,它还能诱导细胞核破碎和活性氧(ROS)的生成,研究人员利用荧光共聚焦成像技术对细胞进行了基于 DAPI、AO 和 DCFH-DA 的染色研究。此外,还研究了 MCDPO 对卷曲杆菌和结核分枝杆菌(Mtb)的抗菌活性。MCDPO 对 Mtb 的最小抑菌浓度(MIC)为 0.39 µg/mL,略优于临床使用的候选药物之一乙胺丁醇。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Synthesis, characterisation, single crystal structure and evaluation of a redox innocent carbazate functionalized phenanthroline for antimycobacterial and anticancer activity
Development of bioactive candidates for cancer and bacterial infections are ever demanding challenges due to the resistance shown by these cancer and bacterial cells for already exposed drug molecules. Methyl carbazate derivatized phenanthroline compound 6-[2-(methoxycarbonyl)diazen-1-yl]-1,10-phenanthroline-5-one (MCDPO) is synthesized in a three step reaction from 1,10-phenanthroline. The spectroscopic features of MCDPO are studied by HRMS, IR, 1H NMR and 13C NMR, UV-visible spectroscopy. The three-dimensional molecular structure of the MCDPO is confirmed by single crystal XRD study. The MCDPO crystallized in a triclinic system P-1 space group with the following unit cell parameter: a = 5.0347(2) Å, b = 10.3509(4) Å, c = 11.8816(4) Å, α = 84.431(3)°, β = 84.172(3)°, γ = 81.380(4)°, and V = 606.92(4) Å3 at T = 133 K. The MCDPO is containing aromatic rings, hydrogen bonding donors as well as hydrogen bonding acceptor groups forming supramolecular molecular arrangements through C-H⋯O, C![]() H⋯N, C-O⋯C, π-π and π⋯C non-covalent interactions. Electrochemical redox and electrochromic features of MCDPO are studied through cyclic voltammetry and UV-vis based spectroelectrochemistry. This compound shows good anticancer activity with IC50 values 1.438, 6.576 and 2.901 µM against 4T1, MCF-7 and PC-3 cancer cell lines respectively. The flow cytometry study on 4T1 cells suggests that the MCDPO promoted cancer cell death by inducing apoptosis, and cell cycle arrest in the G0/G1 phase. It also induces nuclear fragmentation and reactive oxygen species (ROS) generation, which was studied by DAPI, AO, and DCFH-DA based cellular staining studies by fluorescence confocal imaging. The MCDPO also studied for antibacterial activity against Escherichia Coil bacteria and Mycobacterium tuberculosis (Mtb). The MCDPO shows minimum inhibitory concentration (MIC) of 0.39 µg/mL against Mtb which is slightly better than the one of the clinically used drug candidates Ethambutol.
H⋯N, C-O⋯C, π-π and π⋯C non-covalent interactions. Electrochemical redox and electrochromic features of MCDPO are studied through cyclic voltammetry and UV-vis based spectroelectrochemistry. This compound shows good anticancer activity with IC50 values 1.438, 6.576 and 2.901 µM against 4T1, MCF-7 and PC-3 cancer cell lines respectively. The flow cytometry study on 4T1 cells suggests that the MCDPO promoted cancer cell death by inducing apoptosis, and cell cycle arrest in the G0/G1 phase. It also induces nuclear fragmentation and reactive oxygen species (ROS) generation, which was studied by DAPI, AO, and DCFH-DA based cellular staining studies by fluorescence confocal imaging. The MCDPO also studied for antibacterial activity against Escherichia Coil bacteria and Mycobacterium tuberculosis (Mtb). The MCDPO shows minimum inhibitory concentration (MIC) of 0.39 µg/mL against Mtb which is slightly better than the one of the clinically used drug candidates Ethambutol.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


