探索高亲和力铁渗透酶(Ftr1)的Fe3+与REGLE基团之间的相互作用:硅学方法
IF 2.7
4区 生物学
Q2 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
摘要
粘孢子菌病是一种侵袭性真菌感染,在免疫力低下的人群中死亡率很高。由于 COVID-19 大流行,该病最近再次出现,由于缺乏适当的抗真菌药物,患者的预后很差。主要致病菌德莱玛根霉的铁吸收机制对其在人类宿主中的生存和致病至关重要。目前的研究首次聚焦于高亲和力铁渗透酶(Ftr1)的结构动态,而Ftr1是霉菌病的致病因子。Ftr1 是一种跨膜蛋白,在根瘤菌缺铁的条件下负责将 Fe3+ 离子从细胞外环境转运到细胞质。本研究对 Ftr1 进行了三维建模。Ftr1 具有七个跨膜螺旋,N 端和 C 端分别位于胞外和胞内区域。此外,本研究还描述了第四个跨膜螺旋的 REGLE 主题中的谷氨酸残基与 Fe3+ 的相互作用。分子动力学模拟研究显示,该结构基团中的甘氨酸会破坏螺旋的稳定性,从而使 E157 更接近带正电荷的离子。了解 Fe3+ 离子与 Ftr1 之间的相互作用将有助于设计有效的小分子药物来对付这一治疗粘液瘤病的新靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
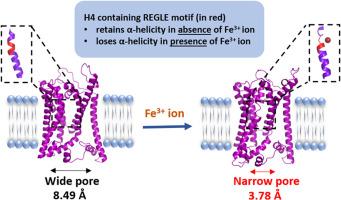
Exploring the interaction between Fe3+ and REGLE motif of the high-affinity iron permease (Ftr1): An in silico approach
Mucormycosis is an invasive fungal infection with high mortality rate in immunocompromised individuals. Due to COVID-19 pandemic, the disease has resurfaced recently and lack of appropriate antifungals resulted in a poor outcome in patients. The iron uptake mechanism in Rhizopus delemar, the predominant causal agent, is crucial for its survival and pathogenesis in human host. The current study is first of its kind to focus on structural dynamics of high affinity iron permease (Ftr1), a virulence factor for Mucormycosis. Ftr1 is a transmembrane protein which is responsible for transport of Fe3+ ion from extracellular milieu to cytoplasm under iron starving conditions in Rhizopus. In this work, the three-dimensional modelling of Ftr1 was carried out. The Ftr1 possessed seven transmembrane helices with N- & C-termini in extracellular and intracellular regions respectively. Moreover, the present study delineates interaction of glutamic acid residues, found in the REGLE motif of fourth transmembrane helix with Fe3+. The molecular dynamics simulation study revealed that the glycine present in the motif destabilizes the helix thereby bringing E157 closer to positively charged ion. Understanding the interaction between Fe3+ ion and Ftr1 would be helpful in designing effective small molecule drugs against this novel therapeutic target for treating mucormycosis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of molecular graphics & modelling
生物-计算机:跨学科应用
CiteScore
5.50
自引率
6.90%
发文量
216
审稿时长
35 days
期刊介绍:
The Journal of Molecular Graphics and Modelling is devoted to the publication of papers on the uses of computers in theoretical investigations of molecular structure, function, interaction, and design. The scope of the journal includes all aspects of molecular modeling and computational chemistry, including, for instance, the study of molecular shape and properties, molecular simulations, protein and polymer engineering, drug design, materials design, structure-activity and structure-property relationships, database mining, and compound library design.
As a primary research journal, JMGM seeks to bring new knowledge to the attention of our readers. As such, submissions to the journal need to not only report results, but must draw conclusions and explore implications of the work presented. Authors are strongly encouraged to bear this in mind when preparing manuscripts. Routine applications of standard modelling approaches, providing only very limited new scientific insight, will not meet our criteria for publication. Reproducibility of reported calculations is an important issue. Wherever possible, we urge authors to enhance their papers with Supplementary Data, for example, in QSAR studies machine-readable versions of molecular datasets or in the development of new force-field parameters versions of the topology and force field parameter files. Routine applications of existing methods that do not lead to genuinely new insight will not be considered.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


