探索热休克蛋白作为帕金森病的治疗靶点。
IF 5.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
帕金森病(PD)的特征是折叠错误的α-突触核蛋白(α-syn)的积累。促进错误折叠蛋白的降解已被证明是缓解帕金森病的有效方法。本综述强调了特定热休克蛋白(HSP)在调节α-syn聚集和神经元存活方面的作用。HSP27可防止糖基化诱导的α-syn聚集,破坏铜离子相互作用,抑制线粒体凋亡,防止多巴胺能神经细胞死亡。HSP70 可通过促进有丝分裂和防止神经元凋亡来减轻多巴胺能神经元损伤。HSC70 在伴侣介导的自噬中发挥关键作用,并促进溶酶体降解。GRP78 可减轻异常蛋白质聚集。HSP70-HSP40-HSP110 系统能够降解 α-syn 淀粉样纤维。抑制 HSP90 的表达可保护神经元。进一步的研究应优先开发 HSPs 的调节剂,以治疗帕金森病。尽管HSPs在帕金森病的治疗中大有可为,但由于其作用复杂,因此有必要谨慎开发治疗方法以挖掘其潜力。了解不同HSPs的特定作用对于开发清除α-syn的有效疗法至关重要。本文章由计算机程序翻译,如有差异,请以英文原文为准。
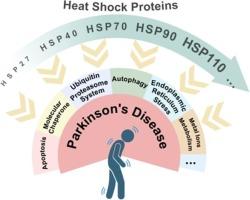
Exploring heat shock proteins as therapeutic targets for Parkinson’s disease
Parkinson’s disease (PD) is characterized by the accumulation of misfolded α-synuclein (α-syn). Promoting the degradation of misfolded proteins has been shown to be an effective approach to alleviate PD. This review highlights the roles of specific heat shock proteins (HSPs) in modulating α-syn aggregation and neuronal survival. HSP27 prevents glycosylation-induced α-syn aggregation, disrupts copper ion interactions, inhibits mitochondrial apoptosis, and prevents dopaminergic neuronal cell death. HSP70 alleviates dopaminergic neuronal damage by promoting mitophagy and preventing neuronal apoptosis. HSC70 plays a critical role in chaperone-mediated autophagy and facilitates lysosomal degradation. GRP78 mitigates abnormal protein aggregation. The HSP70-HSP40-HSP110 system is capable of degrading α-syn amyloid fibers. Inhibition of HSP90 expression protects neurons. Further research should prioritize developing regulators of HSPs as treatments for PD. While HSPs offer promise in PD management, their complex roles necessitate cautious therapeutic development to harness their potential. Understanding the specific roles of different HSPs will be essential to developing effective therapies for α-syn clearance.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biochemical pharmacology
医学-药学
CiteScore
10.30
自引率
1.70%
发文量
420
审稿时长
17 days
期刊介绍:
Biochemical Pharmacology publishes original research findings, Commentaries and review articles related to the elucidation of cellular and tissue function(s) at the biochemical and molecular levels, the modification of cellular phenotype(s) by genetic, transcriptional/translational or drug/compound-induced modifications, as well as the pharmacodynamics and pharmacokinetics of xenobiotics and drugs, the latter including both small molecules and biologics.
The journal''s target audience includes scientists engaged in the identification and study of the mechanisms of action of xenobiotics, biologics and drugs and in the drug discovery and development process.
All areas of cellular biology and cellular, tissue/organ and whole animal pharmacology fall within the scope of the journal. Drug classes covered include anti-infectives, anti-inflammatory agents, chemotherapeutics, cardiovascular, endocrinological, immunological, metabolic, neurological and psychiatric drugs, as well as research on drug metabolism and kinetics. While medicinal chemistry is a topic of complimentary interest, manuscripts in this area must contain sufficient biological data to characterize pharmacologically the compounds reported. Submissions describing work focused predominately on chemical synthesis and molecular modeling will not be considered for review.
While particular emphasis is placed on reporting the results of molecular and biochemical studies, research involving the use of tissue and animal models of human pathophysiology and toxicology is of interest to the extent that it helps define drug mechanisms of action, safety and efficacy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


