探索罗丹宁宇宙:设计和合成基于荧光罗丹宁的衍生物,作为抗α-突触核蛋白和 2N4R tau 的抗纤维化和抗偶联剂。
IF 3.3
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
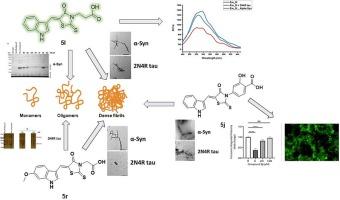
Tau和α-synuclein(α-syn)是容易聚集的蛋白质,它们可能是阿尔茨海默病(AD)、路易体痴呆(LBD)和帕金森病(PD)患者大脑病变的原因。早期的 tau 低聚物和原纤维被认为与人类认知障碍密切相关,而有毒的 α-syn 低聚物则与行为运动障碍有关。因此,同时靶向蛋白聚集体和寡聚体非常具有挑战性。在此,我们设计并合成了基于罗丹宁的化合物,用于靶向 tau 和 α-syn 蛋白的纤维和低聚物。其中,含吲哚的罗丹宁 5l 和 5r 对 α-syn 纤维具有很高的抗聚集活性,能将硫黄素-T(ThT)荧光降低到 5% 以下。此外,当 5r 与非磷酸化的 tau 0N4R 和 2N4R 以及高磷酸化的 tau 异构体 1N4R 共同孵育时,硫黄素-S(ThS)的荧光明显减少。透射电子显微镜(TEM)分析验证了 5l 和 5r 对这两种蛋白聚集体的强大抗纤维活性。此外,在光诱导未修饰蛋白交联(PICUP)试验中,5l 和 5r 都能高度抑制 0N4R tau 和 α-syn 寡聚体的形成。在 510 纳米波长处,5l 的荧光发射强度在两种蛋白纤维存在的情况下几乎被淬灭一半。5r 与 2N4R 纤维素结合时也显示出类似的荧光反应,而与α-syn 聚合体结合时则未观察到淬灭效应。为了证实 5l 和 5r 分解致密纤维的能力,我们使用提取的人体 Aβ 斑块进行了体内分解试验。这两种抑制剂都减少了从AD大脑中分离出的Aβ纤维。5l 和 5r 未能显示出对基于细胞的 α-syn 包涵体形成的活性。然而,另一种吲哚基衍生物 5j 在 5 µM 的浓度下可阻止 α-syn 包涵体的形成。总之,吲哚基-罗丹宁支架可作为进一步结构优化的基石,以获得针对AD、LBD和PD的双靶向疾病修饰候选物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Exploring the rhodanine universe: Design and synthesis of fluorescent rhodanine-based derivatives as anti-fibrillar and anti-oligomer agents against α-synuclein and 2N4R tau
Tau and α-synuclein (α-syn) are prone-to-aggregate proteins that can be responsible for pathological lesions found in the brains of Alzheimer’s disease (AD), Lewy body dementia (LBD), and Parkinson’s disease (PD) patients. The early-stage oligomers and protofibrils of tau are believed to be strongly linked to human cognitive impairment while the toxic α-syn oligomers are associated with behavioral motor deficits. Therefore, concurrent targeting of both proteinaceous aggregates and oligomers are very challenging. Herein, rhodanine-based compounds were designed and synthesized to target the fibrils and oligomers of tau and α-syn proteins. In particular, the indole-containing rhodanines 5l and 5r displayed significantly high anti-aggregation activity towards α-syn fibrils by reducing of the thioflavin-T (ThT) fluorescence to less than 5 %. Moreover, 5r showed a remarkable decrease in the fluorescence of thioflavin-S (ThS) when incubated with the non-phosphorylated tau 0N4R and 2N4R, as well as the hyperphosphorylated tau isoform 1N4R. Transmission electron microscopy (TEM) analyses validated the powerful anti-fibrillar activity of 5l and 5r towards both protein aggregates. In addition, both 5l and 5r highly suppressed 0N4R tau and α-syn oligomer formation using the photo-induced cross-linking of unmodified protein (PICUP) assay. The fluorescence emission intensity of 5l was quenched to almost half in the presence of both protein fibrils at 510 nm. 5r showed a similar fluorescence response upon binding to 2N4R fibrils while no quenching effect was observed with α-syn aggregates. Ex vivo disaggregation assay using extracted human Aβ plaques was employed to confirm the ability of 5l and 5r to disaggregate the dense fibrils. Both inhibitors reduced the Aβ fibrils isolated from AD brains. 5l and 5r failed to show activity toward the cell-based α-syn inclusion formation. However, another indolyl derivative 5j prevented the α-syn inclusion at 5 µM. Collectively, the indolyl-rhodanine scaffold could act as a building block for further structural optimization to obtain dual targeting disease-modifying candidates for AD, LBD, and PD.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioorganic & Medicinal Chemistry
医学-生化与分子生物学
CiteScore
6.80
自引率
2.90%
发文量
413
审稿时长
17 days
期刊介绍:
Bioorganic & Medicinal Chemistry provides an international forum for the publication of full original research papers and critical reviews on molecular interactions in key biological targets such as receptors, channels, enzymes, nucleotides, lipids and saccharides.
The aim of the journal is to promote a better understanding at the molecular level of life processes, and living organisms, as well as the interaction of these with chemical agents. A special feature will be that colour illustrations will be reproduced at no charge to the author, provided that the Editor agrees that colour is essential to the information content of the illustration in question.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


