无定形碳酸钙的稳定和结晶机理。
IF 9.7
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
无定形相在各种应用中大有可为,并被生物广泛用作生产具有复杂形态和优异特性的生物矿物的前体。然而,人们对无定形相的稳定和结晶机制并不完全了解,尤其是在有添加剂存在的情况下。在此,我们以无定形碳酸钙(ACC)为模型系统,系统地研究了不同链长的聚(天冬氨酸)(pAsp)存在时无定形相的结晶途径。结果表明,纯 ACC 通过典型的溶解-再结晶机制转化为方解石和钒钛石的混合物,3% 的 Asp 单体的影响可以忽略不计。然而,链长仅为 10 的 pAsp 能强烈抑制聚集诱导的钒铁矿球体的形成,同时通过经典的离子-离子附着作用略微延迟方解石的生长,从而在动力学上有利于方解石的形成。此外,溶液离子对方解石生长的抑制作用随着 pAsp 链长度或浓度的增加而变得更加突出,这显著提高了无定形相的稳定性,并导致 ACC 伪形态转化为钒钛铁矿纳米颗粒后,通过非经典的颗粒附着机制结晶出球形或细长的方解石。这些结果使我们对添加剂存在下 ACC 的稳定和结晶机理有了更全面的了解,并为控制非晶前驱体结晶过程中晶体的多晶体选择和形态提供了指导。本文章由计算机程序翻译,如有差异,请以英文原文为准。
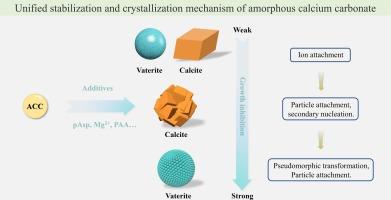
Stabilization and crystallization mechanism of amorphous calcium carbonate
Amorphous phases hold great promise in diverse applications and are widely used by organisms as precursors to produce biominerals with complex morphologies and excellent properties. However, the stabilization and crystallization mechanisms of amorphous phases are not fully understood, especially in the presence of additives. Here, using amorphous calcium carbonate (ACC) as the model system, we systematically investigate the crystallization pathways of amorphous phases in the presence of poly(Aspartic acid) (pAsp) with various chain lengths. Results show that pure ACC transforms into a mixture of calcite and vaterite via the typical dissolution–recrystallization mechanism and 3 % of Asp monomer exhibits negligible effect. However, pAsp with a chain length of only 10 strongly inhibits the aggregation-induced formation of vaterite spheres while slightly delaying the growth of calcite via classical ion-by-ion attachment, thus kinetically favoring the formation of calcite. Moreover, the inhibition effect of calcite growth from solution ions becomes more prominent with the increase of pAsp chain length or concentration, which significantly improves the stability of the amorphous phase and leads to crystallization of spherical or elongated calcite via the nonclassical particle attachment mechanism after pseudomorphic transformation of ACC into vaterite nanoparticles. These results allow us to reach a more comprehensive understanding of the stabilization and crystallization mechanism of ACC in the presence of additives and provide guidelines for controlling the polymorph selection and morphology of crystals during the crystallization of amorphous precursors.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
16.10
自引率
7.10%
发文量
2568
审稿时长
2 months
期刊介绍:
The Journal of Colloid and Interface Science publishes original research findings on the fundamental principles of colloid and interface science, as well as innovative applications in various fields. The criteria for publication include impact, quality, novelty, and originality.
Emphasis:
The journal emphasizes fundamental scientific innovation within the following categories:
A.Colloidal Materials and Nanomaterials
B.Soft Colloidal and Self-Assembly Systems
C.Adsorption, Catalysis, and Electrochemistry
D.Interfacial Processes, Capillarity, and Wetting
E.Biomaterials and Nanomedicine
F.Energy Conversion and Storage, and Environmental Technologies

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


