通过协调巨噬细胞极化和糖酵解,靶向 SphK1/S1PR3 轴可改善败血症诱发的多器官损伤。
IF 4.6
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et biophysica acta. Molecular cell research
Pub Date : 2024-11-14
DOI:10.1016/j.bbamcr.2024.119877
引用次数: 0
摘要
败血症是一种异质性和不精确的疾病,其特点是对感染的异常反应,已被证实对免疫稳态有不利影响。最近,巨噬细胞代谢被认为是开发新型败血症免疫调节疗法的诱人靶点。然而,决定巨噬细胞功能的微调调节因子以及巨噬细胞代谢重编程的具体机制在很大程度上仍然模糊不清。Sphingosine-1-phosphate(S1P)是一种鞘脂分解代谢介质,主要通过鞘氨醇激酶1(SphK1)催化形成,通过与巨噬细胞中表达的S1P受体3(S1PR3)结合介导脓毒症中的炎症。我们在此证明,SphK1/S1PR3 轴在脂多糖(LPS)诱导的巨噬细胞和脓毒症小鼠肺中上调,并级联激活 HIF-1α、HK2 和 PFKFB3 等预溶解信号。PF-543 对 Sphk1 的靶向抑制能有效抑制体外和体内上调的 SphK1/S1PR3 轴。此外,PF-543 还能显著抑制败血症相关炎症和体内多器官损伤。此外,PF-543 不仅能抑制 LPS 处理巨噬细胞中的关键糖酵解酶 HIF-1α、HK2 和 PFKFB3,还能抑制败血症小鼠体内的 HK2 和 PFKFB3。沉默或抑制 SphK1 可抑制促炎性 M1 巨噬细胞,同时增强抗炎性 M2 巨噬细胞。有趣的是,敲除 S1PR3 能有效抑制糖酵解相关标记物,恢复 LPS 调节的 M1/M2 极化,并减轻 NF-κB p65 的激活。总之,我们的研究首次证明了在脓毒症期间,PF-543 以一种依赖于 SphK1/S1PR3 的方式协调了巨噬细胞极化和沃伯格效应的比例失衡,缓解了高炎症和多器官衰竭,为脓毒症的药物治疗增添了一个新的难题。本文章由计算机程序翻译,如有差异,请以英文原文为准。
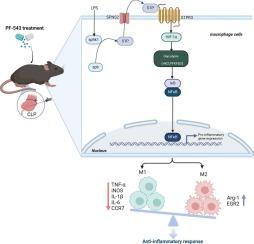
Targeting SphK1/S1PR3 axis ameliorates sepsis-induced multiple organ injury via orchestration of macrophage polarization and glycolysis
Sepsis is a heterogeneous and imprecise disorder characterized by aberrant response to infection which has been accredited for detrimental impact on immune homeostasis. Recently, macrophage metabolism has been recognized as attractive targets to develop novel immunomodulatory therapy for sepsis research. However, the fine-tuning regulators dictating macrophage functions and the specific mechanisms underlying macrophage metabolic reprogramming remain largely obscure. Sphingosine-1-phosphate (S1P), a metabolic mediator of sphingolipid catabolism, predominantly formed through sphingosine kinase 1 (SphK1) catalyzing, mediates inflammation in sepsis by binding to S1P receptor 3 (S1PR3) expressed in macrophages. Here we demonstrate that SphK1/S1PR3 axis was upregulated in lipopolysaccharide (LPS)-induced macrophages and septic mice lungs, cascading the activation of proglycolytic signaling such as HIF-1α, HK2 and PFKFB3. Targeted inhibition of Sphk1 by PF-543 effectively abrogated upregulated SphK1/S1PR3 axis in vitro and in vivo. In addition, PF-543 significantly suppressed sepsis-related inflammation and multi-organ injury in vivo. Furthermore, PF-543 not only blunted key glycolytic enzymes HIF-1α, HK2, and PFKFB3 in LPS-treated macrophages but also inhibited HK2 and PFKFB3 in septic mice. Silencing or inhibiting SphK1 tempered pro-inflammatory M1 macrophages while boosted anti-inflammatory M2 macrophages. Intriguingly, S1PR3 knockdown proficiently dampened glycolysis-associated markers, retrieved LPS-modulated M1/M2 polarization and attenuated NF-κB p65 activation. In conclusion, our study provides the first evidence that PF-543 orchestrates proportional imbalance of macrophage polarization and the Warburg effect in a SphK1/S1PR3 dependent manner during sepsis, mitigating both hyperinflammation and multi-organ failure, adding a novel puzzle piece to pharmacologically exploitable therapy for sepsis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
10.00
自引率
2.00%
发文量
151
审稿时长
44 days
期刊介绍:
BBA Molecular Cell Research focuses on understanding the mechanisms of cellular processes at the molecular level. These include aspects of cellular signaling, signal transduction, cell cycle, apoptosis, intracellular trafficking, secretory and endocytic pathways, biogenesis of cell organelles, cytoskeletal structures, cellular interactions, cell/tissue differentiation and cellular enzymology. Also included are studies at the interface between Cell Biology and Biophysics which apply for example novel imaging methods for characterizing cellular processes.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


