构建高性能水氧化电催化剂的多金属杂原子框架协同战略。
IF 9.4
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
开发用于氧进化反应(OER)的低成本、高活性和非贵金属催化剂意义重大。含有铁、钴和镍的多金属催化剂具有显著的氧进化反应活性,而三元金属催化剂中每种组分的具体贡献和协同效应却一直难以确定。在这项工作中,我们合成了一系列 S 和 N 掺杂的单金属、双金属和三金属空心碳球电催化剂 (M-SNC),目的是提高催化剂的 OER 活性,并阐明各种金属在铁钴镍三元金属催化剂中的独特作用和协同效应。我们的系统分析表明,铁的加入有效降低了过电位,钴加速了 OER 的动力学过程,而镍的加入则进一步提高了 OER 的性能。得益于这些协同效应,FeCoNi-SNC 的过电位低至 270 mV,反应后无形态或结构变化,在 10 mA cm-2 的条件下可维持 72 小时的高活性。此外,组装好的 FeCoNi-SNC ||Pt/C 水电解装置可持续运行 65,000 秒,降解极小,证明了其实际应用的潜力。这项工作提出了一种制备低成本、高效率 OER 催化剂的协同策略,并进一步为合理设计和制备多组分催化剂提供了启示。本文章由计算机程序翻译,如有差异,请以英文原文为准。
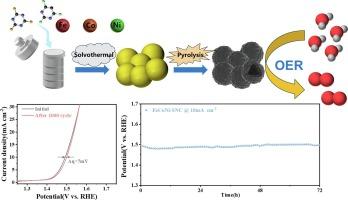
Synergy strategy of multi-metals confined in heteroatom framework toward constructing high-performance water oxidation electrocatalysts
The development of a low-cost, highly active, and non-precious metal catalyst for oxygen evolution reaction (OER) is of great significance. Multi-metallic catalysts containing Fe, Co, and Ni exhibit remarkable OER activity, while the specific contributions of each component and the synergistic effects in the ternary metal catalyst has remained elusive. In this work, we synthesized a series of S and N-doped mono-metallic, bi-metallic, and tri-metallic hollow carbon sphere electrocatalysts (M−SNC) with the goal of enhancing the catalysts OER activity and shedding light on the unique roles and synergistic effects of the various metals in the FeCoNi ternary metal catalyst. Our systematic analyses demonstrated the introduction of Fe effectively reduces the overpotential, Co accelerates the kinetics of OER, and the addition of Ni further improves the OER performance. Benefiting from the synergistic effects, the FeCoNi-SNC exhibits a low overpotential of 270 mV, with no morphological or structural changes after reaction, maintaining high activity for 72 h at 10 mA cm−2. Moreover, the assembled FeCoNi-SNC || Pt/C water electrolysis device operates for 65,000 s with minimal degradation, demonstrating its potential for practical application. This work presents a synergy strategy for the preparation of low-cost and highly efficient OER catalysts and further provides insights into the rational design and preparation of multicomponent catalysts.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
16.10
自引率
7.10%
发文量
2568
审稿时长
2 months
期刊介绍:
The Journal of Colloid and Interface Science publishes original research findings on the fundamental principles of colloid and interface science, as well as innovative applications in various fields. The criteria for publication include impact, quality, novelty, and originality.
Emphasis:
The journal emphasizes fundamental scientific innovation within the following categories:
A.Colloidal Materials and Nanomaterials
B.Soft Colloidal and Self-Assembly Systems
C.Adsorption, Catalysis, and Electrochemistry
D.Interfacial Processes, Capillarity, and Wetting
E.Biomaterials and Nanomedicine
F.Energy Conversion and Storage, and Environmental Technologies

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


