磺胺衍生物化半乳糖苷可选择性地靶向galectin-9N-末端结构域中一个尚未探索的结合位点。
IF 3.3
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
合成了磺酰胺衍生化半乳糖吡喃糖苷的四种方向和位置变体,并针对人半乳糖凝集素-1、-3、-4C(C-端)、-7、-8N(N-端)、-8C(C-端)、-9N(N-端)和-9C(C-端)进行了评估、结果表明,其中一个磺酰胺位点和方向(甲基 3-{4-[2-(苯磺酰氨基)-苯基]-三唑基}-3-脱氧-α-d-吡喃半乳糖苷)与 galectin-9N 的结合亲和力比相应的苯基三唑(缺少苯磺酰胺分子)高 6-15 倍。分子动力学模拟表明,抑制剂采用了一种与 galectin-9N 结合位点互补的构象,其中磺酰胺分子突出到与 A-B 亚位点垂直且低于 A-B 亚位点的一个未开发的非保留结合位点,与 His61 NH 质子相互作用。由此发现的 galectin-9N 抑制剂对其他 galectins 具有前所未有的选择性,从而成为研究 galectin-9 生物功能的宝贵工具。本文章由计算机程序翻译,如有差异,请以英文原文为准。
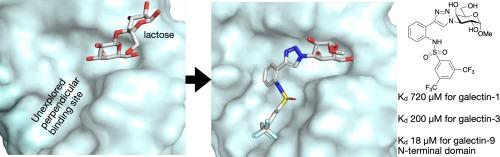
Sulfonamide-derivatized galactosides selectively target an unexplored binding site in the galectin-9N-terminal domain
Four directional and positional variants of sulfonamide-derivatized galactopyranosides were synthesized and evaluated against human galectin-1, -3, -4C (C-terminal), -7, -8N (N-terminal), -8C (C-terminal), -9N (N-terminal), and -9C (C-terminal), which revealed that one of the sulfonamide positions and directionalities (methyl 3-{4-[2-(phenylsulfonylamino)-phenyl]-triazolyl}-3-deoxy-α-d-galactopyranosides) bound with 6–15 fold higher affinity than the corresponding phenyltriazole (lacking the phenylsulfonamide moiety) for galectin-9N. Molecular dynamic simulations suggested that inhibitor adopted a conformation that is complementary to the galectin-9N binding site and where the sulfonamide moiety protrudes into an unexplored and non-conserved binding site perpendicular to and below the A–B subsite to interact with a His61 N![]() H proton. This resulted in the discovery of galectin-9N inhibitors with unprecedented selectivity over other galectins, thus constituting valuable tools for studies of the biological functions of galectin-9.
H proton. This resulted in the discovery of galectin-9N inhibitors with unprecedented selectivity over other galectins, thus constituting valuable tools for studies of the biological functions of galectin-9.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioorganic & Medicinal Chemistry
医学-生化与分子生物学
CiteScore
6.80
自引率
2.90%
发文量
413
审稿时长
17 days
期刊介绍:
Bioorganic & Medicinal Chemistry provides an international forum for the publication of full original research papers and critical reviews on molecular interactions in key biological targets such as receptors, channels, enzymes, nucleotides, lipids and saccharides.
The aim of the journal is to promote a better understanding at the molecular level of life processes, and living organisms, as well as the interaction of these with chemical agents. A special feature will be that colour illustrations will be reproduced at no charge to the author, provided that the Editor agrees that colour is essential to the information content of the illustration in question.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


