以共价和非共价碱基配对相互作用组合为模板的长度和序列选择性聚合物合成
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
信息可以编码并存储在线性合成聚合物的单体单元序列中。序列信息的复制在生物学中具有根本性的重要意义,但对合成聚合物化学来说却是一项挑战。我们采用共价和非共价碱基对的组合,实现了合成聚合物的高保真模板化合成,将信息编码为不同侧链识别单元的序列。使用酯碱基对将对位炔构筑模块连接到模板上,使用氢键碱基对将重氮构筑模块连接到模板上。利用铜催化的叠氮-炔环化反应将模板上的复制链拉长,然后通过水解共价酯碱基对来裂解产生的双链。通过使用具有三个氧化膦或三个 4-硝基苯酚识别单元的识别编码三聚氰胺低聚物来形成非共价碱基对,重氮化物与模板的亲和力特别高,从而使模板化聚合步骤可以在低浓度下进行,相对于竞争性分子间过程,这促进了模板上的分子内反应。在三步反应序列中使用了两种不同的模板(7-聚合体和 11-聚合体),以最小的副反应量获得序列互补的拷贝链。本文章由计算机程序翻译,如有差异,请以英文原文为准。
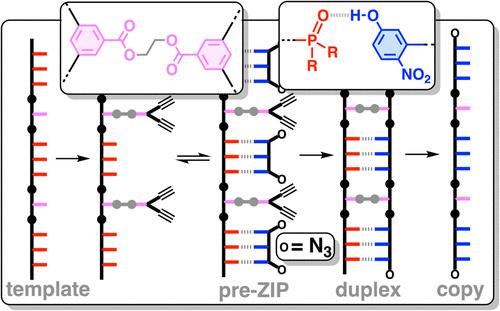
Length and Sequence-Selective Polymer Synthesis Templated by a Combination of Covalent and Noncovalent Base-Pairing Interactions
Information can be encoded and stored in sequences of monomer units organized in linear synthetic polymers. Replication of sequence information is of fundamental importance in biology; however, it represents a challenge for synthetic polymer chemistry. A combination of covalent and noncovalent base pairs has been used to achieve high-fidelity templated synthesis of synthetic polymers that encode information as a sequence of different side-chain recognition units. Dialkyne building blocks were attached to the template by using ester base pairs, and diazide building blocks were attached to the template by using H-bond base pairs. Copper-catalyzed azide–alkyne cycloaddition reactions were used to zip up the copy strand on the template, and the resulting duplex was cleaved by hydrolyzing the covalent ester base pairs. By using recognition-encoded melamine oligomers with either three phosphine oxide or three 4-nitrophenol recognition units to form the noncovalent base pairs, exceptionally high affinities of the diazides for the template were achieved, allowing the templated polymerization step to be carried out at low concentrations, which promoted on-template intramolecular reactions relative to competing intermolecular processes. Two different templates, a 7-mer and an 11-mer, were used in the three-step reaction sequence to obtain the sequence-complementary copy strands with minimal amounts of side reaction.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


