从块体到表面:锂离子电池浓缩乙腈电解质溶解结构的拉曼光谱分析
IF 3.3
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
最近的研究表明,在锂-氧(Li-O2)电池的充放电过程中,浓缩电解质溶液可以提高有机溶剂的稳定性。然而,人们对电解质浓度对电极表面锂离子(Li-ions)溶解结构的影响及其对氧还原和进化反应(ORR/OER)的影响仍然知之甚少。在本研究中,我们利用原位拉曼光谱和表面增强拉曼光谱,研究了锂离子在不同浓度的乙腈(CH3CN)和双(三氟甲烷磺酰)亚胺锂(LiTFSI)电解质中的溶解结构以及在金电极表面的溶解结构。我们的研究结果表明,电解质浓度的增加会减少游离 CH3CN 分子的数量,从而显著改变电极表面的溶解结构。分解的 CH3CN 物种在金电极表面占主导地位,而不可逆的副反应在高浓度电解质中受到抑制。这项研究强调了电解质浓度在优化溶解结构和提高锂-O2 电池电解质稳定性方面的重要性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
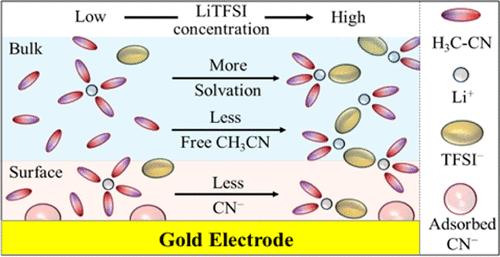
From Bulk to Surface: A Raman Spectroscopic Analysis of Solvation Structures in Concentrated Acetonitrile Electrolytes for Li–O2 Batteries
Recent studies indicate that concentrated electrolyte solutions can enhance the stability of organic solvents during the charge/discharge processes in lithium–oxygen (Li–O2) batteries. However, the effects of electrolyte concentration on the solvation structures of lithium ions (Li-ions) at the electrode surface and their implications for oxygen reduction and evolution reactions (ORR/OER) remain poorly understood. In this study, we investigate the solvation structures of Li-ions in bulk solutions and on a gold electrode surface at various concentrations of acetonitrile (CH3CN) and lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) electrolytes, using in situ Raman and surface-enhanced Raman spectroscopy. Our findings show that increasing electrolyte concentration decreases the number of free CH3CN molecules, significantly altering solvation structures at the electrode surface. Decomposed CH3CN species predominate the gold electrode surface, while the irreversible side reactions are suppressed in highly concentrated electrolytes. This research highlights the importance of electrolyte concentration in optimizing solvation structures and enhancing the electrolyte stability of Li–O2 batteries.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


