水解聚环辛烯以获得线性丙烯酸乙酯共聚物,作为实现聚烯烃功能化的一个步骤
IF 5.1
1区 化学
Q1 POLYMER SCIENCE
引用次数: 0
摘要
为了推进通过脱氢然后官能化实现废弃聚烯烃的聚合物到聚合物升级再循环战略,我们成功报告了部分不饱和聚乙烯的类似物--聚环辛烯(PCOE)的氢酯化。在此,我们将 PCOE 转化为一种线性聚(乙烯-丙烯酸乙酯)(EEA)类似物,其丙烯酸乙酯的掺入量范围(乙烯单元的 0 至 18 摩尔%)为 0 至 18 摩尔%。酯的掺入量由反应时间很好地控制,剩余的 C═C 键随后被氢化。笨重的丙烯酸乙酯基团不会与正交聚乙烯晶体结合,随着官能化程度的增加,结晶度、结晶尺寸和熔化温度都会降低。此外,酯化还能调整动态机械性能,随着官能化程度的提高,玻璃化转变温度和橡胶状态下的存储模量都会降低。本文报告的线性 EEA 类似物具有显著的延伸性(应变达 4000%)和高韧性,可与商用无规和支链 EEA 相媲美。最终,我们成功地将脱氢聚乙烯类似物转化为具有良好机械性能的线性 EEA。本文章由计算机程序翻译,如有差异,请以英文原文为准。
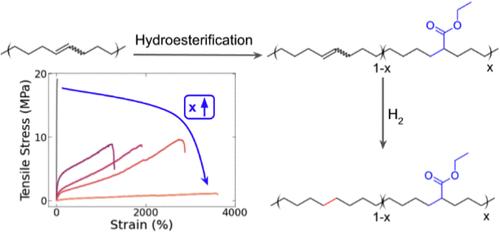
Hydroesterification of Polycyclooctene to Access Linear Ethylene Ethyl Acrylate Copolymers as a Step Toward Polyolefin Functionalization
To advance a strategy of polymer-to-polymer upcycling of waste polyolefin by dehydrogenation then functionalization, we report successful hydroesterification of polycyclooctene (PCOE), an analogue for partially unsaturated polyethylene. Here, we convert PCOE to a linear analog for poly(ethylene-co-ethyl acrylate) (EEA) across a range of ethyl acrylate incorporations (0 to 18 mol % of ethylene units). The ester incorporation was well controlled by reaction time, and the remaining C═C bonds were subsequently hydrogenated. The bulky ethyl acrylate groups did not incorporate into orthorhombic PE crystals, decreasing the crystallinity, crystallite size, and melting temperature with increasing functionalization. Additionally, hydroesterification tuned the dynamic mechanical properties, decreasing both the glass transition temperature and the storage modulus in the rubbery regime with greater functionalization. The linear EEA analogs reported here achieve remarkable extensibility (strain > 4000%) and high toughness, comparable to commercial random and branched EEA. Ultimately, we demonstrate successful conversion of an analogue to dehydrogenated PE to a linear EEA with favorable mechanical properties.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Macromolecules
工程技术-高分子科学
CiteScore
9.30
自引率
16.40%
发文量
942
审稿时长
2 months
期刊介绍:
Macromolecules publishes original, fundamental, and impactful research on all aspects of polymer science. Topics of interest include synthesis (e.g., controlled polymerizations, polymerization catalysis, post polymerization modification, new monomer structures and polymer architectures, and polymerization mechanisms/kinetics analysis); phase behavior, thermodynamics, dynamic, and ordering/disordering phenomena (e.g., self-assembly, gelation, crystallization, solution/melt/solid-state characteristics); structure and properties (e.g., mechanical and rheological properties, surface/interfacial characteristics, electronic and transport properties); new state of the art characterization (e.g., spectroscopy, scattering, microscopy, rheology), simulation (e.g., Monte Carlo, molecular dynamics, multi-scale/coarse-grained modeling), and theoretical methods. Renewable/sustainable polymers, polymer networks, responsive polymers, electro-, magneto- and opto-active macromolecules, inorganic polymers, charge-transporting polymers (ion-containing, semiconducting, and conducting), nanostructured polymers, and polymer composites are also of interest. Typical papers published in Macromolecules showcase important and innovative concepts, experimental methods/observations, and theoretical/computational approaches that demonstrate a fundamental advance in the understanding of polymers.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


