可扩展的无保护基团 Resibufogenin 和 Bufalin 全合成技术
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
我们开发出了一种化学合成方法,可通过七个步骤合成雷西布苷元和布法林,且无需保护基团。从雄烯二酮(AD)开始,通过羟化酶 P-450lun 直接在 C14 处引入一个 α-OH,并进一步将其用作氢化的定向基团,以在铃木交叉偶联后完全控制 C17 构型的 β 方向。14α-OH脱水后进行环氧化反应,得到瑞舒呋甙元。同时,还通过具有挑战性的厌氧 Mukaiyama 水合反应获得了布法林。本文章由计算机程序翻译,如有差异,请以英文原文为准。
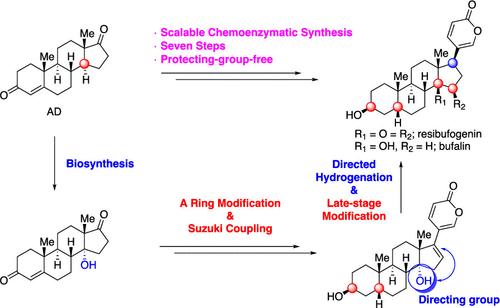
Scalable Protecting-Group-Free Total Synthesis of Resibufogenin and Bufalin
A chemoenzymatic synthesis access to resibufogenin and bufalin was developed in seven steps without protecting groups. Starting with androstenedione (AD), an α-OH was introduced directly at C14 by hydroxylase P-450lun, which was further used as the directing group for hydrogenation to fully control the C17 configuration in the β-orientation after Suzuki cross-coupling. Dehydration of 14α-OH followed by an epoxidation delivered resibufogenin. Simultaneously, bufalin was also obtained via a challenging anaerobic Mukaiyama hydration.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


