Melittin 通过调节 HSF1/PDK3 来抑制有氧糖酵解,从而提高 NSCLC 的化疗敏感性。
IF 4.2
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
非小细胞肺癌(NSCLC)虽然被认为是非免疫原性的,但在临床患者的治疗过程中往往对化疗药物产生抗药性。蜜蜂毒液的主要成分美利汀(C131H229N39O31,CAS:20449-79-0)是一种很有前景的抗癌药物。然而,蜜蜂毒素逆转NSCLC细胞化疗耐药性的机制尚不清楚。本研究利用细胞计数试剂盒8、乙炔基脱氧尿苷检测法和其他检测方法来阐明美利汀对细胞增殖的影响。蛋白质组学、肺癌(LC)组织芯片和 Western 印迹分析用于确定美利汀的潜在靶点。采用A549/DDP细胞研究美利汀对顺铂耐药性的影响。此外,还进行了体内动物实验,以进一步明确美利汀对A549/DDP细胞顺铂耐药性的调控功能。结果表明,美利汀通过下调丙酮酸脱氢酶激酶3(PDK3)介导的有氧糖酵解和抑制热休克因子1(HSF1)的表达,抑制了A549/DDP细胞的恶性进展。美利汀与 KNK437 联用可增强治疗效果,并通过逆转有氧糖酵解作用削弱 A549/DDP 细胞的化疗耐药性。体内实验证实,美利汀提高了A549/DDP细胞对顺铂的敏感性。这些数据表明,美利汀通过调节HSF1/PDK3抑制有氧糖酵解,从而提高了A549/DDP细胞对顺铂的敏感性。它可能为临床NSCLC患者的化疗耐药提供一种新的治疗方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
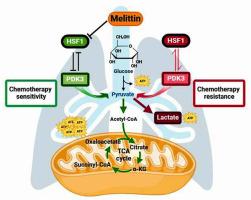
Melittin suppresses aerobic glycolysis by regulating HSF1/PDK3 to increase chemosensitivity of NSCLC
Non-small cell lung cancer (NSCLC), although considered non-immunogenic, is often resistant to chemotherapy agents during the course of treatment in clinical patients. Melittin (C131H229N39O31, CAS: 20449-79-0), the major component of honey bee venom, is a promising anticancer drug. However, the mechanism employed by melittin to reverse chemotherapy resistance of NSCLC cells remains unknown. In this study, the Cell Counting Kit 8, ethynyl deoxyuridine assay, and other assays were utilized to elucidate the melittin effects upon cell proliferation. Proteomics, lung cancer (LC) tissue chip, and Western blot analysis were used to identify potential targets of melittin. A549/DDP cells were employed to investigate the melittin effects against cisplatin resistance. Also, an in vivo animal experiment was conducted to further clarify the regulatory function of melittin towards cisplatin resistance of A549/DDP cells. Results showed that melittin inhibited malignant progression of A549/DDP cells by down-regulation of pyruvate dehydrogenase kinase 3 (PDK3)-mediated aerobic glycolysis and inhibition of heat shock factor 1 (HSF1) expression. The therapeutic effect of melittin was increased by combination with KNK437 and impaired chemotherapy resistance regarding A549/DDP cells via reversing aerobic glycolysis. The in vivo experiments confirmed that melittin incremented A549/DDP cell cisplatin sensitivities. Collectively, the data suggested that melittin suppressed aerobic glycolysis by regulating HSF1/PDK3, which incremented cisplatin sensitivity of A549/DDP cells. It may provide a new treatment method for chemotherapy resistance in clinical NSCLC patients.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.00
自引率
0.00%
发文量
572
审稿时长
34 days
期刊介绍:
The European Journal of Pharmacology publishes research papers covering all aspects of experimental pharmacology with focus on the mechanism of action of structurally identified compounds affecting biological systems.
The scope includes:
Behavioural pharmacology
Neuropharmacology and analgesia
Cardiovascular pharmacology
Pulmonary, gastrointestinal and urogenital pharmacology
Endocrine pharmacology
Immunopharmacology and inflammation
Molecular and cellular pharmacology
Regenerative pharmacology
Biologicals and biotherapeutics
Translational pharmacology
Nutriceutical pharmacology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


