跨解剖尺度深度学习中基于结构的不确定性:白质病变分割分析。
IF 7
2区 医学
Q1 BIOLOGY
引用次数: 0
摘要
本文以多发性硬化症(MS)患者的磁共振成像(MRI)扫描白质病变(WML)分割为背景,探讨了不确定性量化(UQ)作为自动深度学习(DL)工具可信度指标的问题。我们的研究侧重于结构化输出分割任务中不确定性的两个主要方面。首先,我们假定可靠的不确定性度量应指出不确定性值高的预测可能是错误的。其次,我们研究了在不同解剖尺度(体素、病灶或患者)上量化不确定性的优点。我们假设每个尺度的不确定性都与特定类型的错误有关。我们的研究旨在通过对域内和域外设置进行单独分析来证实这种关系。我们在方法学方面的主要贡献是:(i) 根据结构预测差异,开发了量化病变和患者尺度不确定性的新方法;(ii) 扩展了误差保留曲线分析框架,以方便评估病变和患者尺度的 UQ 性能。来自 444 名患者的多中心 MRI 数据集的结果表明,与平均体素尺度不确定值的测量方法相比,我们提出的测量方法能更有效地捕捉病变和患者尺度的模型误差。我们在 https://github.com/Medical-Image-Analysis-Laboratory/MS_WML_uncs 上提供了 UQ 协议代码。本文章由计算机程序翻译,如有差异,请以英文原文为准。
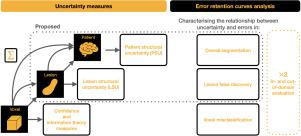
Structural-based uncertainty in deep learning across anatomical scales: Analysis in white matter lesion segmentation
This paper explores uncertainty quantification (UQ) as an indicator of the trustworthiness of automated deep-learning (DL) tools in the context of white matter lesion (WML) segmentation from magnetic resonance imaging (MRI) scans of multiple sclerosis (MS) patients. Our study focuses on two principal aspects of uncertainty in structured output segmentation tasks. First, we postulate that a reliable uncertainty measure should indicate predictions likely to be incorrect with high uncertainty values. Second, we investigate the merit of quantifying uncertainty at different anatomical scales (voxel, lesion, or patient). We hypothesize that uncertainty at each scale is related to specific types of errors. Our study aims to confirm this relationship by conducting separate analyses for in-domain and out-of-domain settings. Our primary methodological contributions are (i) the development of novel measures for quantifying uncertainty at lesion and patient scales, derived from structural prediction discrepancies, and (ii) the extension of an error retention curve analysis framework to facilitate the evaluation of UQ performance at both lesion and patient scales. The results from a multi-centric MRI dataset of 444 patients demonstrate that our proposed measures more effectively capture model errors at the lesion and patient scales compared to measures that average voxel-scale uncertainty values. We provide the UQ protocols code at https://github.com/Medical-Image-Analysis-Laboratory/MS_WML_uncs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Computers in biology and medicine
工程技术-工程:生物医学
CiteScore
11.70
自引率
10.40%
发文量
1086
审稿时长
74 days
期刊介绍:
Computers in Biology and Medicine is an international forum for sharing groundbreaking advancements in the use of computers in bioscience and medicine. This journal serves as a medium for communicating essential research, instruction, ideas, and information regarding the rapidly evolving field of computer applications in these domains. By encouraging the exchange of knowledge, we aim to facilitate progress and innovation in the utilization of computers in biology and medicine.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


