电化学镍催化 C(sp2)-C(sp3)交叉亲电偶联中牺牲阳极的非无辜作用
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
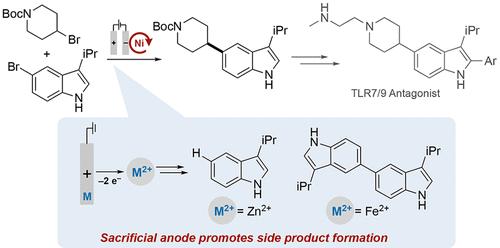
由锌、铁和镁等廉价金属组成的人工阳极被广泛用于支持电化学镍催化的交叉亲电偶联(XEC)反应,以及其他还原性电化学转化。这类阳极之所以吸引人,是因为它们能提供稳定的反电极电位,而且通常能避免干扰还原化学反应。本研究概述了电化学镍催化 XEC 反应的开发过程,该反应简化了关键药物中间体的获取过程。然而,牺牲阳极氧化产生的金属离子会直接导致化学和电化学镍催化 XEC 反应中常见的均偶联和原脱卤副产品。使用分层电池可限制阳极衍生金属离子的干扰,并支持高产率,副产物的形成可忽略不计,从而引入了一种克服镍催化 XEC 主要局限性之一的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Non-Innocent Role of Sacrificial Anodes in Electrochemical Nickel-Catalyzed C(sp2)–C(sp3) Cross-Electrophile Coupling
Sacrificial anodes composed of inexpensive metals such as Zn, Fe, and Mg are widely used to support electrochemical nickel-catalyzed cross-electrophile coupling (XEC) reactions, in addition to other reductive electrochemical transformations. Such anodes are appealing because they provide a stable counter-electrode potential and typically avoid interference with the reductive chemistry. The present study outlines the development of an electrochemical Ni-catalyzed XEC reaction that streamlines access to a key pharmaceutical intermediate. Metal ions derived from sacrificial anode oxidation, however, directly contribute to homocoupling and proto-dehalogenation side products that are commonly formed in chemical and electrochemical Ni-catalyzed XEC reactions. Use of a divided cell limits interference by the anode-derived metal ions and supports a high product yield with negligible side product formation, introducing a strategy to overcome one of the main limitations of Ni-catalyzed XEC.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


