MAPK-SOX2通路的重新激活使KRASG12C抑制剂耐药的肿瘤对铁中毒敏感。
IF 10.7
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
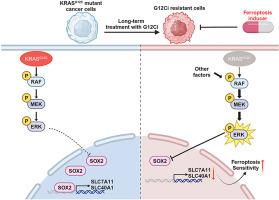
包括 AMG510 和 MRTX849 在内的 KRASG12C 抑制剂(G12Ci)的临床成功受到了获得性耐药性发展的限制。当务之急是找到一种新型有效的治疗方法来逆转或靶向这种耐药性。为此,我们建立了G12Ci(AMG510和MRTX849)耐药的KRASG12C突变癌细胞系,并用FDA批准的药物库进行筛选。我们发现,索拉非尼和拉帕替尼等铁突变诱导剂对 G12Ci 耐药细胞有明显的生长抑制作用。从机理上讲,G12Ci耐药细胞表现出MAPK信号的重新激活,从而抑制了SOX2介导的胱氨酸转运体SLC7A11和铁排出体SLC40A1的表达。因此,细胞内 GSH 含量低而铁含量高使这些耐药肿瘤对铁变态反应诱导剂过敏。异位过表达SOX2或SLC7A11和SLC40A1可使G12Ci耐药细胞对铁突变产生抗性。磺胺沙拉嗪(SAS)诱导的铁变态反应对AMG510耐药的KRASG12C突变细胞异种移植的肿瘤生长有明显的抑制作用。总之,我们的研究结果表明,用铁蛋白诱导剂治疗对G12Ci耐药的癌症患者是一种新的治疗策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Reactivation of MAPK-SOX2 pathway confers ferroptosis sensitivity in KRASG12C inhibitor resistant tumors
The clinical success of KRASG12C inhibitors (G12Ci) including AMG510 and MRTX849 is limited by the eventual development of acquired resistance. A novel and effective treatment to revert or target this resistance is urgent. To this end, we established G12Ci (AMG510 and MRTX849) resistant KRASG12C mutant cancer cell lines and screened with an FDA-approved drug library. We found the ferroptosis inducers including sorafenib and lapatinib stood out with an obvious growth inhibition in the G12Ci resistant cells. Mechanistically, the G12Ci resistant cells exhibited reactivation of MAPK signaling, which repressed SOX2-mediated expression of cystine transporter SLC7A11 and iron exporter SLC40A1. Consequently, the low intracellular GSH level but high iron content engendered hypersensitivity of these resistant tumors to ferroptosis inducers. Ectopic overexpression of SOX2 or SLC7A11 and SLC40A1 conferred resistance to ferroptosis in the G12Ci resistant cells. Ferroptosis induced by sulfasalazine (SAS) achieved obvious inhibition on the tumor growth of xenografts derived from AMG510-resistant KRASG12C-mutant cells. Collectively, our results suggest a novel therapeutic strategy to treat patients bearing G12Ci resistant cancers with ferroptosis inducers.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Redox Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-
CiteScore
19.90
自引率
3.50%
发文量
318
审稿时长
25 days
期刊介绍:
Redox Biology is the official journal of the Society for Redox Biology and Medicine and the Society for Free Radical Research-Europe. It is also affiliated with the International Society for Free Radical Research (SFRRI). This journal serves as a platform for publishing pioneering research, innovative methods, and comprehensive review articles in the field of redox biology, encompassing both health and disease.
Redox Biology welcomes various forms of contributions, including research articles (short or full communications), methods, mini-reviews, and commentaries. Through its diverse range of published content, Redox Biology aims to foster advancements and insights in the understanding of redox biology and its implications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


