整合多组学数据揭示肿瘤微环境中的神经母细胞瘤亚型
IF 5.2
2区 医学
Q1 MEDICINE, RESEARCH & EXPERIMENTAL
引用次数: 0
摘要
神经母细胞瘤(NB)是一种严重的儿科肿瘤,起源于发育中的交感神经系统,临床表现多种多样,包括自发消退和侵袭性转移。这种差异性表明存在不同的神经母细胞瘤亚型,因此需要对其进行准确分类,以便进行有效的靶向治疗。在这项研究中,我们采用了相似性网络融合(SNF)算法,确定了三种NB亚型,包括间质样(MES)、MYCN样(MYCN)和神经原样(Neurogenic)。MES 亚型的免疫相关通路激活程度最高。MYCN 亚型的预后最差,细胞生长和增殖通路富集。相反,神经源亚型的预后最好,富含交感神经系统发育过程。通过单细胞RNA测序(scRNA-seq)分析,我们研究了这些不同NB亚型的肿瘤微环境,发现了MYCN亚型和神经源亚型中肾上腺素能细胞的不同分化轨迹。我们还在 MES 亚型中发现了大量的幼稚 T 细胞,以及与 MES 和 MYCN 亚型中观察到的独特可塑性相关的间质细胞亚型。药物敏感性预测分析表明,MES亚型可能对MEK抑制剂有良好反应,而MYCN亚型则可能对Bcl-2抑制剂敏感。我们的综合多组学方法能将 NB 精确地分层为生物学上不同的亚型,从而促进亚型特异性治疗策略的开发,改善患者管理和生存预后。本文章由计算机程序翻译,如有差异,请以英文原文为准。
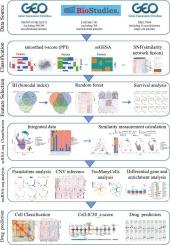
Integrating multi-omics data reveals neuroblastoma subtypes in the tumor microenvironment
Neuroblastoma (NB) is a severe pediatric tumor originating from the developing sympathetic nervous system, characterized by diverse clinical outcomes, including spontaneous regression and aggressive metastasis. This variability suggests the existence of different NB subtypes, necessitating accurate classification for effective targeted treatment. In this study, we employed the similarity network fusion (SNF) algorithm and identified three NB subtypes, including mesenchymal-like (MES), MYCN-like (MYCN), and neurogenic-like (Neurogenic). The MES subtype exhibited the highest activation of immune-related pathways. The MYCN subtype demonstrated the worst prognosis, with enrichment in cell growth and proliferation pathways. Conversely, the Neurogenic subtype showed the best prognosis, with enrichment in sympathetic nervous system development processes. Through single-cell RNA sequencing (scRNA-seq) analysis, we examined the tumor microenvironments of these distinct NB subtypes, revealing divergent differentiation trajectories for adrenergic cells within the MYCN and Neurogenic subtypes. We also identified a significant presence of naïve T cells in the MES subtype, as well as mesenchymal cell subtypes associated with the unique plasticity observed in both the MES and MYCN subtypes. Drug sensitivity prediction analysis suggested that the MES subtype may respond favorably to MEK inhibitors, while the MYCN subtype may be susceptible to Bcl-2 inhibitors. Our integrative multi-omics approach enabled precise stratification of NB into biologically distinct subtypes, potentially facilitating the development of subtype-specific therapeutic strategies for improved patient management and survival outcomes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Life sciences
医学-药学
CiteScore
12.20
自引率
1.60%
发文量
841
审稿时长
6 months
期刊介绍:
Life Sciences is an international journal publishing articles that emphasize the molecular, cellular, and functional basis of therapy. The journal emphasizes the understanding of mechanism that is relevant to all aspects of human disease and translation to patients. All articles are rigorously reviewed.
The Journal favors publication of full-length papers where modern scientific technologies are used to explain molecular, cellular and physiological mechanisms. Articles that merely report observations are rarely accepted. Recommendations from the Declaration of Helsinki or NIH guidelines for care and use of laboratory animals must be adhered to. Articles should be written at a level accessible to readers who are non-specialists in the topic of the article themselves, but who are interested in the research. The Journal welcomes reviews on topics of wide interest to investigators in the life sciences. We particularly encourage submission of brief, focused reviews containing high-quality artwork and require the use of mechanistic summary diagrams.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


