次要人参皂苷总量通过抗氧化、抗炎、调节肠道微生物群和血清代谢发挥抗疲劳作用。
IF 5.2
2区 医学
Q1 MEDICINE, RESEARCH & EXPERIMENTAL
引用次数: 0
摘要
小人参皂苷具有显著的抗疲劳能力。本研究旨在探讨通过益生菌发酵和高压蒸汽处理工艺提取的总小头人参皂甙(TMGs)的抗疲劳机制。研究人员利用负重游泳法在 BALB/c 雄性小鼠中建立了疲劳模型,并以 200 毫克/千克的剂量连续四周口服 TMGs。通过评估肝脏氧化应激、骨骼肌炎症指标以及对肠道微生物群和血清代谢的影响,探讨了 TMGs 的抗疲劳机制。结果表明,TMGs能通过调节PGC-1α/KEAP1/NRF2/HO-1途径,显著提高SOD、CAT、ATP和Na+-K+-ATP酶的水平,增强抗氧化能力。同时,TMGs 还能降低炎症因子 TNF-α、IL-1β 和 IL-6 的水平,并通过调节 AMPK/TORC2/CREB/PGC-1α 通路抑制炎症。TMGs还能调节肠道微生物群,增加益生菌的丰度和盲肠中短链脂肪酸(SCFAs)的含量。血清代谢组学分析表明,TMGs 能显著影响疲劳模型小鼠的血清代谢谱,通过影响抗疲劳相关的代谢途径来调节代谢标志物。总之,TMGs 通过抗氧化和抗炎作用发挥了显著的抗疲劳作用,并通过调节肠道微生物群和血清代谢缓解疲劳。本文章由计算机程序翻译,如有差异,请以英文原文为准。
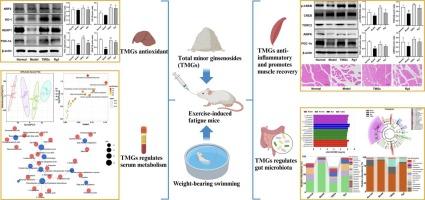
Total minor ginsenosides exert anti-fatigue effects via antioxidant, anti-inflammatory, regulating gut microbiota and serum metabolism
Minor ginsenosides have demonstrated notable anti-fatigue capabilities. The aim of this study was to investigate the anti-fatigue mechanisms of total minor ginsenosides (TMGs) derived from a process involving probiotic fermentation and high-pressure steam treatment. The fatigue model was established in BALB/c male mice using weight-bearing swimming and TMGs were administered by orally at a dosage of 200 mg/kg for four weeks. The anti-fatigue mechanisms of TMGs were explored by assessing liver oxidative stress, skeletal muscle inflammation markers, as well as their impact on gut microbiota and serum metabolism. The results indicated that TMGs could significantly increase the levels of SOD, CAT, ATP and Na+-K+-ATPase and enhance the antioxidant capacity by modulating the PGC-1α/KEAP1/NRF2/HO-1 pathway. Meanwhile, TMGs reducing the levels of inflammatory factors TNF-α, IL-1β and IL-6 and inhibited inflammation by modulating the AMPK/TORC2/CREB/PGC-1α pathway. TMGs also regulated the gut microbiota, increasing the abundance of probiotic bacteria and the content of short-chain fatty acids (SCFAs) in the cecum. Serum metabolomics analyses have shown that TMGs can significantly affect the serum metabolic profile of fatigue model mice, regulating metabolic markers through affecting anti-fatigue-related metabolic pathways. In conclusion, TMGs exerted significant anti-fatigue effects through antioxidant and anti-inflammatory effects, and alleviate fatigue by regulating gut microbiota and serum metabolism.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Life sciences
医学-药学
CiteScore
12.20
自引率
1.60%
发文量
841
审稿时长
6 months
期刊介绍:
Life Sciences is an international journal publishing articles that emphasize the molecular, cellular, and functional basis of therapy. The journal emphasizes the understanding of mechanism that is relevant to all aspects of human disease and translation to patients. All articles are rigorously reviewed.
The Journal favors publication of full-length papers where modern scientific technologies are used to explain molecular, cellular and physiological mechanisms. Articles that merely report observations are rarely accepted. Recommendations from the Declaration of Helsinki or NIH guidelines for care and use of laboratory animals must be adhered to. Articles should be written at a level accessible to readers who are non-specialists in the topic of the article themselves, but who are interested in the research. The Journal welcomes reviews on topics of wide interest to investigators in the life sciences. We particularly encourage submission of brief, focused reviews containing high-quality artwork and require the use of mechanistic summary diagrams.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


