低温电子显微镜相板图像显示出意想不到的试样明显损伤程度。
IF 2.7
3区 生物学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
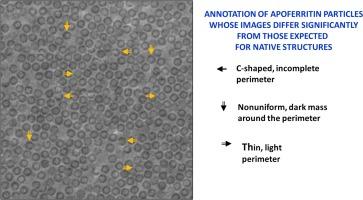
载脂蛋白(apoF)通常被用作单颗粒电子冷冻显微镜(cryo-EM)的测试样本,因为它能持续生成分辨率为 3 Å 或更高的密度图。然而,当我们用激光相位板(LPP)对apoF进行成像时,我们发现图像中颗粒与颗粒之间的差异比我们之前想象的要严重得多。同样,我们发现核酮糖双磷酸羧化酶/加氧酶(rubisco)的图像也表现出比预期大得多的异质性。通过与模拟图像的比较,我们验证了异质性不是由 LPP 的已知特征、拍摄噪音或颗粒方向差异所造成的。我们还证明,根据使用最终重建分辨率作为评估指标的方法,我们的试样与之前文献中使用的试样具有可比性。所有这些都让我们假设,异质性是由于试样净化或网格制备过程中发生的损坏造成的。然而,我们的目标并不是解释异质性的原因;相反,我们报告说,使用 LPP 使明显的损坏变得过于明显,以至于无法忽视。事后看来,在其他人用 Volta 相板记录的 apoF 和 20S 蛋白酶体的图像中也能看到类似的异质性。因此,我们得出结论:相位板图像(在低空间频率下)对比度的提高,也使我们有可能在单粒子基础上观察到以前未被注意到的各种形式的生物功能异质性结构。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Cryo-EM phase-plate images reveal unexpected levels of apparent specimen damage
Apoferritin (apoF) is commonly used as a test specimen in single-particle electron cryo-microscopy (cryo-EM), since it consistently produces density maps that go to 3 Å resolution or higher. When we imaged apoF with a laser phase plate (LPP), however, we observed more severe particle-to-particle variation in the images than we had previously thought to exist. Similarly, we found that images of ribulose bisphosphate carboxylase/oxygenase (rubisco) also exhibited a much greater amount of heterogeneity than expected. By comparison to simulations of images, we verified that the heterogeneity is not explained by the known features of the LPP, shot noise, or differences in particle orientation. We also demonstrate that our specimens are comparable to those previously used in the literature, based on using the final-reconstruction resolution as the metric for evaluation. All of this leads us to the hypothesis that the heterogeneity is due to damage that has occurred either during purification of the specimen or during preparation of the grids. It is not, however, our goal to explain the causes of heterogeneity; rather, we report that using the LPP has made the apparent damage too obvious to be ignored. In hindsight, similar heterogeneity can be seen in images of apoF and the 20S proteasome which others had recorded with a Volta phase plate. We therefore conclude that the increased contrast of phase-plate images (at low spatial frequencies) should also make it possible to visualize, on a single-particle basis, various forms of biologically functional heterogeneity in structure that had previously gone unnoticed.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of structural biology
生物-生化与分子生物学
CiteScore
6.30
自引率
3.30%
发文量
88
审稿时长
65 days
期刊介绍:
Journal of Structural Biology (JSB) has an open access mirror journal, the Journal of Structural Biology: X (JSBX), sharing the same aims and scope, editorial team, submission system and rigorous peer review. Since both journals share the same editorial system, you may submit your manuscript via either journal homepage. You will be prompted during submission (and revision) to choose in which to publish your article. The editors and reviewers are not aware of the choice you made until the article has been published online. JSB and JSBX publish papers dealing with the structural analysis of living material at every level of organization by all methods that lead to an understanding of biological function in terms of molecular and supermolecular structure.
Techniques covered include:
• Light microscopy including confocal microscopy
• All types of electron microscopy
• X-ray diffraction
• Nuclear magnetic resonance
• Scanning force microscopy, scanning probe microscopy, and tunneling microscopy
• Digital image processing
• Computational insights into structure
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


