二甲双胍通过AMPK介导的GDF15诱导作用抑制非小细胞肺癌细胞的迁移和上皮细胞向间质转化。
IF 4.7
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
生长分化因子15(GDF15)可作为二甲双胍的生物标志物,它介导二甲双胍的减重作用。然而,GDF15是否也是二甲双胍抑制癌变的分子靶点,目前仍是一个未知数。本研究探讨了GDF15在二甲双胍对非小细胞肺癌(NSCLC)细胞的抗癌作用中的作用和分子机制。我们发现,二甲双胍能显著抑制NSCLC A549和NCI-H460细胞的迁移,并减少上皮细胞向间质转化(EMT)相关分子的表达,包括神经粘附素(N-cadherin)、基质金属蛋白酶2(MMP2)和锌指转录因子Snail,但增加了上皮粘附素(E-cadherin)的表达。此外,二甲双胍增加了GDF15及其上游转录因子活化转录因子4(ATF4)和C/EBP同源蛋白(CHOP)的表达,并增加了NSCLC细胞中AMP激活蛋白激酶(AMPK)的磷酸化。GDF15 siRNA能部分逆转二甲双胍对NSCLC细胞迁移的抑制作用。此外,二甲双胍诱导的GDF15、CHOP和ATF4表达的增加以及迁移的抑制在使用特异性AMPK抑制剂化合物C处理后被部分逆转。同时,二甲双胍能显著抑制裸鼠体内 NCI-H460 异种移植瘤的生长,增加 GDF15 的表达,并调节异种移植瘤中 EMT 和迁移相关蛋白的表达。总之,我们的研究结果提供了新的见解,揭示了二甲双胍对NSCLC的抗癌作用是由AMPK激活介导的,因此GDF15可以作为二甲双胍的潜在分子靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
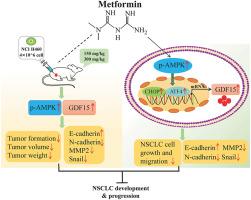
Metformin inhibits migration and epithelial-to-mesenchymal transition in non-small cell lung cancer cells through AMPK-mediated GDF15 induction
The growth differentiation factor 15 (GDF15) may serve as a biomarker of metformin, which mediates the bodyweight lowering effect of metformin. However, whether GDF15 also serves as a molecular target of metformin to inhibit carcinogenesis remains largely unknown. This study examined the role and molecular mechanisms of GDF15 in the anticancer effects of metformin in non-small cell lung cancer (NSCLC) cells, which has never been reported before. We found that metformin significantly inhibited the migration of NSCLC A549 and NCI-H460 cells and reduced the expression of epithelial-to-mesenchymal transition (EMT)-related molecules, including neuro-cadherin (N-cadherin), matrix metalloproteinase 2 (MMP2), and the zinc finger transcription factor Snail, but increased epithelial cadherin (E-cadherin) expression. Furthermore, metformin increased GDF15 and its upstream transcription factors activated transcription factor 4 (ATF4) and C/EBP-homologous protein (CHOP) expressions and increased AMP-activated protein kinase (AMPK) phosphorylation in NSCLC cells. GDF15 siRNA partially reverses the inhibitory effect of metformin on NSCLC cell migration. Moreover, metformin-induced increases in GDF15, CHOP, and ATF4 expression and the inhibition of migration were partially reversed by treatment with Compound C, a specific AMPK inhibitor. Meanwhile, metformin significantly inhibited NCI-H460 xenograft tumor growth in nude mice, increased GDF15 expression, and regulated EMT- and migration-related protein expression in xenograft tumors. In conclusion, our results provide novel insights into revealing that GDF15 can serve as a potential molecular target of metformin owing to its anti-cancer effect in NSCLC, which is mediated by AMPK activation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.00
自引率
0.00%
发文量
572
审稿时长
34 days
期刊介绍:
The European Journal of Pharmacology publishes research papers covering all aspects of experimental pharmacology with focus on the mechanism of action of structurally identified compounds affecting biological systems.
The scope includes:
Behavioural pharmacology
Neuropharmacology and analgesia
Cardiovascular pharmacology
Pulmonary, gastrointestinal and urogenital pharmacology
Endocrine pharmacology
Immunopharmacology and inflammation
Molecular and cellular pharmacology
Regenerative pharmacology
Biologicals and biotherapeutics
Translational pharmacology
Nutriceutical pharmacology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


