胰高血糖素样肽-1受体激动剂 Exendin-4 通过 PKA 途径扩张血管和抗重塑调节动脉导管。
IF 4.2
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
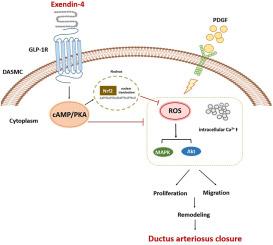
动脉导管(DA)关闭的机制包括血管收缩和血管重塑。之前的研究结果表明,胰高血糖素样肽-1 受体激动剂(GLP-1RA)在肺循环中具有降压和抗重塑作用。然而,它在DA中的作用仍然未知。本研究旨在探讨一种 GLP-1RA --- exendin-4(Ex-4)是否能调节肺动脉瓣的通畅,并阐明其作用机制。在确认体内新生大鼠DA组织中存在GLP-1R后,我们依次研究了Ex-4对新生大鼠DA通畅性的影响。出生两小时后,我们观察到对照组大鼠的 DA 自发闭合。与此相反,Ex-4 可阻止 DA 闭合,同时减少内膜增厚。Ex-4 可减轻体内离体 DA 环的氧诱导血管收缩。在使用 PKA 抑制剂 H89 的情况下,这种作用会减弱。在体外,Ex-4 可抑制血小板衍生生长因子(PDGF)-BB 诱导的 DA 平滑肌细胞的增殖和迁移。此外,Ex-4 还抑制了 PDGF-BB 诱导的活性氧(ROS)产生、钙动员以及 MAPK 和 Akt 通路的信号转导。此外,Ex-4 还保留了被 PDGF-BB 削弱的 Nrf2 核表达。同样,Ex-4 的所有这些体外效应都被 H89 削弱。总之,Ex-4通过PKA途径扩张血管和抗重塑,维持了出生后DA的通畅。在临床治疗中,GLP-1R/PKA通路有望成为DA通畅的靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Exendin-4, a glucagon-like peptide-1 receptor agonist, regulates ductus arteriosus by vasodilation and anti-remodeling through the PKA pathway
The mechanisms of ductus arteriosus (DA) closure involve vasoconstriction and vascular remodeling. Previous findings indicate that the glucagon-like peptide-1 receptor agonist (GLP-1RA) exhibits antihypertensive and anti-remodeling effects in the pulmonary circulation. However, its role in the DA remains unknown. This study aimed to investigate whether exendin-4 (Ex-4), a GLP-1RA, can regulate DA patency and elucidate its mechanisms. After confirming the presence of GLP-1R in neonatal rat DA tissue in vivo, the effects of Ex-4 on DA patency in neonatal rats were sequentially examined. Two hours after birth, we observed spontaneous closure of the DA in control rats. In contrast, Ex-4 prevented the closure of DA, accompanied by reduced intimal thickening. Ex-4 attenuated oxygen-induced vasoconstriction in isolated DA rings ex vivo. This effect was diminished in the presence of H89, a PKA inhibitor. In vitro, Ex-4 inhibited platelet-derived growth factor (PDGF)-BB-induced proliferation and migration of DA smooth muscle cells. Additionally, Ex-4 inhibited PDGF-BB-induced reactive oxygen species (ROS) production, calcium mobilization, and signal transduction of MAPK and Akt pathways. Furthermore, Ex-4 preserved the nuclear expression of Nrf2 attenuated by PDGF-BB. Similarly, all these in vitro effects of Ex-4 were blunted by H89. In conclusion, Ex-4 maintains postnatal DA patency through vasodilatation and anti-remodeling via the PKA pathway. The GLP-1R/PKA pathway emerges as a promising target of DA patency in clinical management.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.00
自引率
0.00%
发文量
572
审稿时长
34 days
期刊介绍:
The European Journal of Pharmacology publishes research papers covering all aspects of experimental pharmacology with focus on the mechanism of action of structurally identified compounds affecting biological systems.
The scope includes:
Behavioural pharmacology
Neuropharmacology and analgesia
Cardiovascular pharmacology
Pulmonary, gastrointestinal and urogenital pharmacology
Endocrine pharmacology
Immunopharmacology and inflammation
Molecular and cellular pharmacology
Regenerative pharmacology
Biologicals and biotherapeutics
Translational pharmacology
Nutriceutical pharmacology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


