通过调节内皮-间充质转化,下调热休克蛋白27可减轻异丙肾上腺素诱导的心肌纤维化和舒张功能障碍。
IF 5.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
心力衰竭(HF)是继发于心脏功能损害的终末期临床综合征,严重影响患者的生活质量和长期预后。心肌纤维化会导致收缩和舒张功能障碍,并促进心力衰竭的恶化。为了改善心脏功能,已经开展了多项有关调节心肌纤维化的研究。热休克蛋白 27(HSP27)是一种小型伴侣蛋白,在细胞应激状态下会过度表达。HSP27 可调节上皮-间质转化,在多种纤维化疾病的病理过程中发挥关键作用。然而,它与心肌纤维化调控的关系尚不清楚。本研究旨在探讨HSP27调控心肌纤维化的机制。我们创建了心脏特异性 HSP25(人类 HSP27 的鼠直向同源物)基因敲除小鼠,并通过超声心动图、组织学和 Western bloting 发现,HSP25 基因敲除抑制了内皮-间质转化(EndMT),减轻了心肌纤维化,并改善了异丙托肾上腺素诱导的高频小鼠的舒张功能障碍。在体外,HSP27敲除可减轻转化生长因子β诱导的内膜种植,而HSP27过表达则可促进内膜种植。此外,研究还发现SMAD3/SNAIL1通路对HSP27介导的EndMT调控至关重要。作为内膜移植调控和心肌纤维化调节的重要分子,HSP27有望成为心房颤动患者的治疗靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
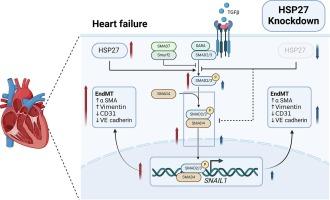
Heat shock protein 27 downregulation attenuates isoprenaline-induced myocardial fibrosis and diastolic dysfunction by modulating the endothelial-mesenchymal transition
Heart failure (HF), an end-stage clinical syndrome secondary to cardiac impairment, significantly affects patients’ quality of life and long-term prognosis. Myocardial fibrosis leads to systolic and diastolic dysfunction, and promotes the progression of HF. Several studies involving the modulation of myocardial fibrosis have been conducted in an effort to improve cardiac function. Heat shock protein 27 (HSP27) is a small chaperone protein that is overexpressed in cellular stress states. HSP27 modulates epithelial-mesenchymal transition, playing a crucial role in the pathology of several fibrotic diseases. However, its association with myocardial fibrosis regulation is unknown. This study aimed to investigate the mechanisms by which HSP27 regulates myocardial fibrosis. We created cardiac-specific HSP25 (the murine ortholog of human HSP27) knockout mice and found that HSP25 knockdown inhibited endothelial-mesenchymal transition (EndMT), attenuated myocardial fibrosis, and ameliorated diastolic dysfunction in isoproterenol-induced HF mice via echocardiography, histology, and western bloting. In vitro, HSP27 knockdown attenuated transforming growth factor beta-induced EndMT, whereas HSP27 overexpression promoted EndMT. Furthermore, the SMAD3/SNAIL1 pathway was found to be crucial for HSP27-mediated EndMT regulation. As an essential molecule in EndMT regulation and myocardial fibrosis modulation, HSP27 may hold promise as a therapeutic target for patients with HF.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biochemical pharmacology
医学-药学
CiteScore
10.30
自引率
1.70%
发文量
420
审稿时长
17 days
期刊介绍:
Biochemical Pharmacology publishes original research findings, Commentaries and review articles related to the elucidation of cellular and tissue function(s) at the biochemical and molecular levels, the modification of cellular phenotype(s) by genetic, transcriptional/translational or drug/compound-induced modifications, as well as the pharmacodynamics and pharmacokinetics of xenobiotics and drugs, the latter including both small molecules and biologics.
The journal''s target audience includes scientists engaged in the identification and study of the mechanisms of action of xenobiotics, biologics and drugs and in the drug discovery and development process.
All areas of cellular biology and cellular, tissue/organ and whole animal pharmacology fall within the scope of the journal. Drug classes covered include anti-infectives, anti-inflammatory agents, chemotherapeutics, cardiovascular, endocrinological, immunological, metabolic, neurological and psychiatric drugs, as well as research on drug metabolism and kinetics. While medicinal chemistry is a topic of complimentary interest, manuscripts in this area must contain sufficient biological data to characterize pharmacologically the compounds reported. Submissions describing work focused predominately on chemical synthesis and molecular modeling will not be considered for review.
While particular emphasis is placed on reporting the results of molecular and biochemical studies, research involving the use of tissue and animal models of human pathophysiology and toxicology is of interest to the extent that it helps define drug mechanisms of action, safety and efficacy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


