PX-478 通过下调 GBE1 抑制 PI3K/AKT/mTOR 通路,从而诱导缺氧条件下急性髓性白血病患者的细胞凋亡。
IF 5.6
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
急性髓性白血病(AML)是一种高度异质性的血液系统恶性肿瘤,其特点是治疗方案有限且有明显的复发倾向。PX-478是一种新型低氧诱导因子1-α(HIF-1α)抑制剂,已在多种癌症模型中显示出抗肿瘤活性,但其在急性髓性白血病中的具体作用仍有待探索。本研究旨在探索PX-478诱导AML细胞凋亡的潜在靶点和机制。首先,在体外缺氧条件下,PX-478通过调节Bcl-2家族和激活线粒体介导的caspase cascade诱导AML细胞凋亡,表现出浓度依赖性效应。此外,体内给药 PX-478 能显著抑制小鼠皮下急性髓细胞白血病异种移植的生长,同时增加肿瘤细胞的凋亡。RNA 测序和细胞研究显示,PX-478 处理的细胞中 PI3K/AKT/mTOR 信号通路下调。同样,细胞研究也表明 PI3K/AKT/mTOR 通路与 PX-478 诱导的 AML 细胞凋亡有关。此外,通过对 RNA 测序差异基因的筛选和随后的实验验证,糖原分支酶 1(GBE1)可能参与了 PX-478 诱导的 AML 细胞凋亡。我们发现,抑制 AML 细胞中 GBE1 的表达(siGBE1)会导致 PI3K/AKT/mTOR 通路下调并诱导细胞凋亡。在使用减少了 GBE1 表达(shGBE1)的 AML 细胞进行的实验中,PX-478 处理并未进一步下调通路或增强细胞凋亡。在 shGBE1 细胞中重新表达 GBE1 可缓解细胞凋亡,减少 PX-478 诱导的细胞凋亡和通路下调。总之,我们的研究结果提供了令人信服的证据,证明PX-478通过抑制AML细胞中的GBE1,抑制PI3K/AKT/mTOR通路,从而诱导细胞凋亡。本文章由计算机程序翻译,如有差异,请以英文原文为准。
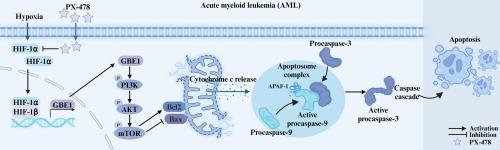
PX-478 induces apoptosis in acute myeloid leukemia under hypoxia by inhibiting the PI3K/AKT/mTOR pathway through downregulation of GBE1
Acute myeloid leukemia (AML) is a highly heterogeneous hematologic malignancy characterized by limited therapeutic options and a pronounced tendency for relapse. PX-478, a novel inhibitor of hypoxia-inducible factor 1-alpha (HIF-1α), has demonstrated antitumor activity across various cancer models, but its specific role in AML remains unexplored. This study aimed to explore the potential target and mechanism of PX-478-induced AML cell apoptosis. First, PX-478 induced AML cell apoptosis in vitro under hypoxia via modulation of the Bcl-2 family and activation of the mitochondria-mediated caspase cascade, exhibiting a concentration-dependent effect. Additionally, in vivo administration of PX-478 led to notable inhibition of subcutaneous AML xenograft growth in mice, coupled with increased tumor cell apoptosis. RNA sequencing and cellular studies revealed downregulation of the PI3K/AKT/mTOR signaling pathway in PX-478-treated cells. Consistently, cellular studies also implicated PI3K/AKT/mTOR pathway in PX-478-induced AML cell apoptosis. Furthermore, by screening for RNA sequencing differential genes and subsequent experimental verification, Glycogen branching enzyme 1 (GBE1) may be involved in PX-478-induced apoptosis in AML cells. We found that inhibiting GBE1 expression in AML cells (siGBE1) led to downregulation of the PI3K/AKT/mTOR pathway and induced apoptosis. In experiments using AML cells with reduced GBE1 expression (shGBE1), PX-478 treatment did not further downregulate the pathway or enhance apoptosis. Re-expression of GBE1 in shGBE1 cells alleviated apoptosis and reduced PX-478- induced apoptosis and pathway downregulation. In conclusion, our findings provide convincing evidence that PX-478 induces apoptosis by inhibiting the PI3K/AKT/mTOR pathway through downregulation of GBE1 in AML cells.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biochemical pharmacology
医学-药学
CiteScore
10.30
自引率
1.70%
发文量
420
审稿时长
17 days
期刊介绍:
Biochemical Pharmacology publishes original research findings, Commentaries and review articles related to the elucidation of cellular and tissue function(s) at the biochemical and molecular levels, the modification of cellular phenotype(s) by genetic, transcriptional/translational or drug/compound-induced modifications, as well as the pharmacodynamics and pharmacokinetics of xenobiotics and drugs, the latter including both small molecules and biologics.
The journal''s target audience includes scientists engaged in the identification and study of the mechanisms of action of xenobiotics, biologics and drugs and in the drug discovery and development process.
All areas of cellular biology and cellular, tissue/organ and whole animal pharmacology fall within the scope of the journal. Drug classes covered include anti-infectives, anti-inflammatory agents, chemotherapeutics, cardiovascular, endocrinological, immunological, metabolic, neurological and psychiatric drugs, as well as research on drug metabolism and kinetics. While medicinal chemistry is a topic of complimentary interest, manuscripts in this area must contain sufficient biological data to characterize pharmacologically the compounds reported. Submissions describing work focused predominately on chemical synthesis and molecular modeling will not be considered for review.
While particular emphasis is placed on reporting the results of molecular and biochemical studies, research involving the use of tissue and animal models of human pathophysiology and toxicology is of interest to the extent that it helps define drug mechanisms of action, safety and efficacy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


