四种纳米抗体作为人类钙传感受体正异构调节剂的详细功能表征。
IF 5.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
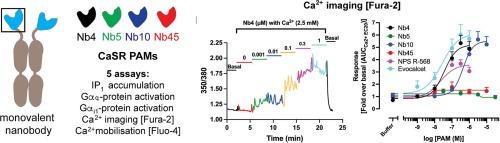
钙传感受体(CaSR)在钙平衡中起着关键作用,CaSR 的小分子和多肽正性异位调节剂(PAMs),即所谓的降钙药,被用于治疗甲状旁腺功能亢进症和低钙血症。在这项研究中,对四种单价纳米抗体(代表具有 CaSR PAM 活性的四个不同纳米抗体家族)在受体上进行了详细的药理学分析。在 Gαq 和 Gαi1 蛋白激活试验以及 Ca2+/Fluo-4 试验中,Nb5 在所有试验中对 CaSR 的 PAM 活性都微乎其微,而 Nb4、Nb10 和 Nb45 都能通过在 HEK293 或 HEK293T 细胞中表达的 myc 表位标记的 CaSR 有效地增强 Ca2+ 诱导的信号传导。在 Ca2+/Fura-2 成像检测中,Nb4 和 Nb10 在稳定的 CaSR-HEK293 细胞系中也显示出相似的 PAM 特性,但令人惊讶的是,在 Ca2+/Fura-2 和 Ca2+/Fluo-4 检测中,Nb45 在该细胞系中完全没有活性。对 Nb45 活性的这种二元差异进行研究后发现,纳米抗体只对 N 端标记了各种表位(myc、HA、Flag-SNAP)的 CaSR 具有调节活性,而对未标记的野生型受体则没有活性。总之,总体而言,每种纳米抗体在一系列检测中都表现出相似的 CaSR PAM 特性,因此它们都没有显示出作为调节剂的途径偏差。然而,在这四种纳米抗体中,Nb4 和 Nb10 可以作为野生型 CaSR 的药理工具,而 Nb45 在未标记的 CaSR 上完全没有活性,这提醒我们,即使认为受体的表位标记在功能上是沉默的,也会对配体的发现工作产生深远的影响。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Detailed functional characterization of four nanobodies as positive allosteric modulators of the human calcium-sensing receptor
The calcium-sensing receptor (CaSR) plays a key role in calcium homeostasis, and small-molecule and peptide positive allosteric modulators (PAMs) of CaSR, so-called calcimimetics, are used in the treatment of hyperparathyroidism and hypocalcemic disorders. In this study, four monovalent nanobodies − representing four distinct nanobody families with CaSR PAM activity − were subjected to elaborate pharmacological profiling at the receptor. While Nb5 displayed negligible PAM activity at CaSR in all assays, Nb4, Nb10 and Nb45 all potently potentiated Ca2+-evoked signalling through a myc epitope-tagged CaSR expressed in HEK293 or HEK293T cells in Gαq and Gαi1 protein activation assays and in a Ca2+/Fluo-4 assay. Nb4 and Nb10 also displayed comparable PAM properties at a stable CaSR-HEK293 cell line in a Ca2+/Fura-2 imaging assay, but surprisingly Nb45 was completely inactive at this cell line in both the Ca2+/Fura-2 and Ca2+/Fluo-4 assays. Investigations into this binary difference in Nb45 activity revealed that the nanobody only possesses modulatory activity at CaSRs tagged N-terminally with various epitopes (myc, HA, Flag-SNAP), whereas it is inactive at the untagged wild-type receptor. In conclusion, overall each of the four nanobodies exhibit similar CaSR PAM properties in a range of assays, and thus none of them display pathway bias as modulators. However, of the four nanobodies Nb4 and Nb10 would be applicable as pharmacological tools for the wild-type CaSR, whereas the complete inactivity of Nb45 at the untagged CaSR serves as an reminder that epitope-tagging of a receptor, even if deemed functionally silent, can have profound implications for ligand discovery efforts.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biochemical pharmacology
医学-药学
CiteScore
10.30
自引率
1.70%
发文量
420
审稿时长
17 days
期刊介绍:
Biochemical Pharmacology publishes original research findings, Commentaries and review articles related to the elucidation of cellular and tissue function(s) at the biochemical and molecular levels, the modification of cellular phenotype(s) by genetic, transcriptional/translational or drug/compound-induced modifications, as well as the pharmacodynamics and pharmacokinetics of xenobiotics and drugs, the latter including both small molecules and biologics.
The journal''s target audience includes scientists engaged in the identification and study of the mechanisms of action of xenobiotics, biologics and drugs and in the drug discovery and development process.
All areas of cellular biology and cellular, tissue/organ and whole animal pharmacology fall within the scope of the journal. Drug classes covered include anti-infectives, anti-inflammatory agents, chemotherapeutics, cardiovascular, endocrinological, immunological, metabolic, neurological and psychiatric drugs, as well as research on drug metabolism and kinetics. While medicinal chemistry is a topic of complimentary interest, manuscripts in this area must contain sufficient biological data to characterize pharmacologically the compounds reported. Submissions describing work focused predominately on chemical synthesis and molecular modeling will not be considered for review.
While particular emphasis is placed on reporting the results of molecular and biochemical studies, research involving the use of tissue and animal models of human pathophysiology and toxicology is of interest to the extent that it helps define drug mechanisms of action, safety and efficacy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


