激活的小胶质细胞分泌组和促炎细胞因子会增加神经元μ-阿片受体的信号传导和表达。
IF 5.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
由于μ-阿片受体在依赖μ-阿片受体功能的过程中可能发挥作用,研究μ-阿片受体(MOR)、神经炎症和神经胶质细胞之间关系的势头日益强劲。传统上,MOR 的激活与免疫抑制有关,但最近的研究结果表明,MOR 与免疫系统之间存在着更加微妙的双向关系。为了进一步研究这种关系,我们在本文中研究了活化的小胶质细胞分泌组和促炎细胞因子在神经元 MOR 表达和信号传导中的作用。我们的研究结果表明,小胶质细胞分泌物和特定细胞因子都会增加神经元 MOR 的表达,并增强[D-Ala2, N-MePhe4, Gly-ol]-enkephalin (DAMGO)诱导的 MOR 激活。我们还发现,DAMGO 诱导的神经炎症会增加神经元 MOR 的表达、激活和调节。我们的研究结果表明,小胶质细胞活化、细胞因子释放和神经元 MOR 动态之间存在反馈回路。未来的研究应深入探讨这种关系的时间动态和功能影响,特别是与吗啡和芬太尼等临床相关的阿片类药物和疼痛治疗。本文章由计算机程序翻译,如有差异,请以英文原文为准。
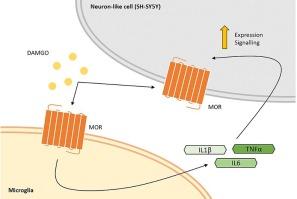
Activated microglia secretome and proinflammatory cytokines increase neuronal mu-opioid receptor signalling and expression
Due to its potential role in processes which rely on mu-opioid receptor function, investigating the relationship between Mu-Opioid receptors (MORs), neuroinflammation, and glial cells has gained momentum. Traditionally, MOR activation has been associated with immunosuppression, but recent findings suggest a more nuanced, bidirectional relationship with the immune system. To further investigate this relationship, herein, we investigated the role of the activated microglia secretome and proinflammatory cytokines in neuronal MOR expression and signalling. Our results show that both microglial secretome and specific cytokines increase neuronal MOR expression and enhance the [D-Ala2, N-MePhe4, Gly-ol]-enkephalin (DAMGO)-induced MOR activation. We also show that DAMGO-induced neuroinflammation increases neuronal MOR expression, activation, and regulation. Our findings suggest a feedback loop between microglial activation, cytokine release, and neuronal MOR dynamics. Future research should delve into the temporal dynamics and functional implications of this relationship, particularly concerning clinically relevant opioids like morphine and fentanyl and pain management.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biochemical pharmacology
医学-药学
CiteScore
10.30
自引率
1.70%
发文量
420
审稿时长
17 days
期刊介绍:
Biochemical Pharmacology publishes original research findings, Commentaries and review articles related to the elucidation of cellular and tissue function(s) at the biochemical and molecular levels, the modification of cellular phenotype(s) by genetic, transcriptional/translational or drug/compound-induced modifications, as well as the pharmacodynamics and pharmacokinetics of xenobiotics and drugs, the latter including both small molecules and biologics.
The journal''s target audience includes scientists engaged in the identification and study of the mechanisms of action of xenobiotics, biologics and drugs and in the drug discovery and development process.
All areas of cellular biology and cellular, tissue/organ and whole animal pharmacology fall within the scope of the journal. Drug classes covered include anti-infectives, anti-inflammatory agents, chemotherapeutics, cardiovascular, endocrinological, immunological, metabolic, neurological and psychiatric drugs, as well as research on drug metabolism and kinetics. While medicinal chemistry is a topic of complimentary interest, manuscripts in this area must contain sufficient biological data to characterize pharmacologically the compounds reported. Submissions describing work focused predominately on chemical synthesis and molecular modeling will not be considered for review.
While particular emphasis is placed on reporting the results of molecular and biochemical studies, research involving the use of tissue and animal models of human pathophysiology and toxicology is of interest to the extent that it helps define drug mechanisms of action, safety and efficacy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


