基于机制的 sirtuin 5 灭活剂:结构-活性关系重点研究。
IF 2.5
4区 医学
Q3 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
Sirtuin 5(SIRT5)是一种赖氨酸脱酰酶,它能裂解由短二羧酸产生的带负电荷的ε-N-酰基赖氨酸翻译后修饰。抑制 SIRT5 被认为是治疗白血病和乳腺癌的一个靶点。在这项工作中,我们进行了一项重点结构-活性关系研究,发现了 SIRT5 的强效抑制剂。通过动力学评估,这些抑制剂的例子被证明是基于机制的失活剂。抑制剂中一个关键的羧基官能团被掩蔽,从而产生了原药,这些原药被证明能与细胞中的 SIRT5 结合。这项工作强调了酶抑制剂动力学表征的重要性,并为进一步优化具有体内应用潜力的 SIRT5 抑制剂提供了启示。本文章由计算机程序翻译,如有差异,请以英文原文为准。
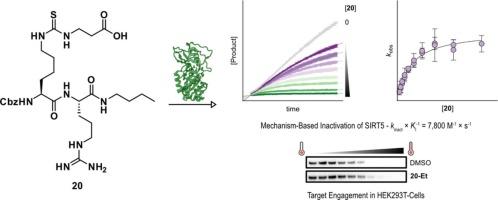
Mechanism-based inactivators of sirtuin 5: A focused structure–activity relationship study
Sirtuin 5 (SIRT5) is a lysine deacylase enzyme that cleaves negatively charged ε-N-acyllysine posttranslational modifications, arising from short dicarboxylic acids. Inhibition of SIRT5 has been suggested as a target for treatment of leukemia and breast cancer. In this work, we performed a focused structure–activity relationship study that identified highly potent inhibitors of SIRT5. Examples of these inhibitors were shown by kinetic evaluation to function as mechanism-based inactivators. Masking of a crucial carboxylate functionality in the inhibitors provided prodrugs, which were demonstrated to bind SIRT5 in cells. This work underscores the importance of kinetic characterization of enzyme inhibitors and provides insights for the further optimization of inhibitors of SIRT5 with potential for in vivo applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
5.70
自引率
3.70%
发文量
463
审稿时长
27 days
期刊介绍:
Bioorganic & Medicinal Chemistry Letters presents preliminary experimental or theoretical research results of outstanding significance and timeliness on all aspects of science at the interface of chemistry and biology and on major advances in drug design and development. The journal publishes articles in the form of communications reporting experimental or theoretical results of special interest, and strives to provide maximum dissemination to a large, international audience.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


