舒林酸与 NO 协同作用的发现扩大了非甾体抗炎药在心脏并发症中的应用。
IF 3.3
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
心脏炎症是心脏功能障碍的病理基石,会引发舒张功能障碍,导致心肌严重缺氧。这种缺氧状态是扩大炎症介质释放的有效刺激,最终限制了单一疗法的治疗潜力。为了应对这一挑战,我们合成了将非甾体抗炎药(NSAID)与一氧化氮(NO)供体结合在一起的化合物。体外筛选揭示出一种舒林酸衍生物具有显著的抗缺氧性炎症损伤活性,尽管与其他非甾体抗炎药物衍生物相比,它对 COX-2 的抑制效果并不理想。这一发现促使我们深入研究舒林酸与 NO 之间错综复杂的协同作用关系。利用双向方差分析和高通量筛选技术,我们无可争议地证实了它们之间的协同作用。此外,我们还通过检测信号分子释放水平和 Western 印迹分析揭示了其潜在机制。最后,我们利用再现心脏炎症和缺氧的大鼠模型,评估了舒林酸及其衍生物在体内的治疗效果。我们的研究结果预示着一种很有前景的候选药物,并为在错综复杂的心血管疾病中扩大非甾体抗炎药的应用奠定了基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
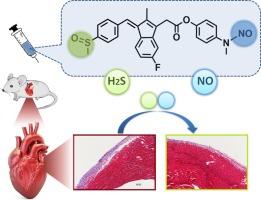
Discovery of the synergy between sulindac and NO expands the application of NSAIDs in cardiac complications
Cardiac inflammation, a pathological cornerstone in cardiac dysfunction, triggers diastolic impairment, leading to profound myocardial hypoxia. This hypoxic state serves as a potent stimulus for the amplification of inflammatory mediator release, ultimately limiting the therapeutic potential of monotherapies. To address this challenge, compounds that integrate non-steroidal anti-inflammatory drugs (NSAIDs) with nitric oxide (NO) donors was synthesized. The in vitro screening unveiled the remarkable anti-hypoxic inflammatory injury activity of a sulindac derivative, despite its suboptimal COX-2 inhibition when comparing with other NSAIDs derivatives. This revelation propelled us to delve deeper into the intricate synergistic relationship between sulindac and NO. Employing two-way ANOVA and high-throughput screening technique, we incontrovertibly confirmed their synergism. Further, the underlying mechanism was unmasked through the detection of signal molecule release levels and western blot analysis. In a definitive step, we evaluated the therapeutic effects of sulindac and its derivatives in vivo, leveraging a rat model that recapitulates cardiac inflammation coupled with hypoxia. Our findings herald a promising drug candidate and establish a foundation for the expanded utilization of NSAIDs in the intricate landscape of cardiovascular diseases.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioorganic & Medicinal Chemistry
医学-生化与分子生物学
CiteScore
6.80
自引率
2.90%
发文量
413
审稿时长
17 days
期刊介绍:
Bioorganic & Medicinal Chemistry provides an international forum for the publication of full original research papers and critical reviews on molecular interactions in key biological targets such as receptors, channels, enzymes, nucleotides, lipids and saccharides.
The aim of the journal is to promote a better understanding at the molecular level of life processes, and living organisms, as well as the interaction of these with chemical agents. A special feature will be that colour illustrations will be reproduced at no charge to the author, provided that the Editor agrees that colour is essential to the information content of the illustration in question.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


