基于喹啉和香豆素的配体及其三羰基铼(I)配合物:合成、光谱特征和对 T 细胞淋巴瘤的抗增殖活性。
IF 3.8
2区 化学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
通过喹啉和香豆素与(E)-表芳樟醛肟的 O-烷基化以及随后与[Re(CO)5Cl]的 O-烷基化,合成了具有醛肟醚连接吡啶分子的新型 6-取代 2-(三氟甲基)喹啉 5a-5e 和香豆素 6a-6d 配体,并通过核磁共振、单晶 X 射线衍射、红外光谱和紫外可见光谱对这些配体进行了全面表征、单晶 X 射线衍射、红外光谱和紫外可见光谱对其进行了全面表征。喹啉和香豆素配体及其三羰基铼(I)配合物对多种人类肿瘤细胞系的抗增殖性评价结果,包括急性淋巴细胞白血病(CCRF-CEM)、急性单核细胞白血病(THP1)、宫颈腺癌(HeLa)、结肠腺癌(HeLa)和胰腺癌(HeLa)、在对结肠腺癌(CaCo-2)、T 细胞淋巴瘤(HuT78)和非肿瘤人成纤维细胞(BJ)的研究中发现,喹啉复合物 5aRe-5eRe 比香豆素复合物 6aRe-6dRe 具有更高的抑制活性,尤其是对 T 细胞淋巴瘤(HuT78)细胞。研究发现,6-甲氧基-2-(三氟甲基)喹啉 5e 和 6-甲基香豆素 6d,以及它们的三羰基铼络合物 5eRe 和 6dRe 能使处于 G0/G1 期的细胞显著聚集,并使处于 G2/M 期的细胞数量明显减少,从而阻滞 HuT78 细胞的细胞周期。与未处理的细胞和用 5e 和 6d 处理的细胞相比,这些铼(I)三羰基复合物还能轻微增加 ROS 的产生,并显著降低线粒体膜电位 50%(5eRe)和 45%(6dRe)。这些结果表明,这些化合物的细胞毒性作用是由它们对线粒体膜电位的影响以及随后的 ROS 生成增加所介导的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
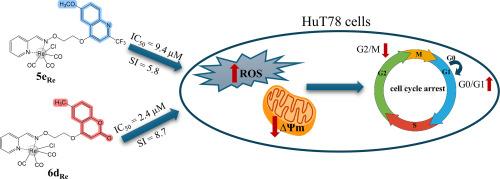
Quinoline- and coumarin-based ligands and their rhenium(I) tricarbonyl complexes: synthesis, spectral characterization and antiproliferative activity on T-cell lymphoma
Novel 6-substituted 2-(trifluoromethyl)quinoline 5a–5e and coumarin 6a–6d ligands with aldoxime ether linked pyridine moiety were synthesized by O-alkylation of quinoline and coumarin with (E)-picolinaldehyde oxime and subsequently with [Re(CO)5Cl] gave rhenium(I) tricarbonyl complexes 5aRe–5eRe and 6aRe–6dRe that were fully characterized by NMR, single-crystal X-ray diffraction, IR and UV–Vis spectroscopy. The results of antiproliferative evaluation of quinoline and coumarin ligands and their rhenium(I) tricarbonyl complexes on various human tumor cell lines, including acute lymphoblastic leukemia (CCRF-CEM), acute monocytic leukemia (THP1), cervical adenocarcinoma (HeLa), colon adenocarcinoma (CaCo-2), T-cell lymphoma (HuT78), and non-tumor human fibroblasts (BJ) showed that the quinoline complexes 5aRe–5eRe had higher inhibitory activity than coumarin complexes 6aRe–6dRe, particularly against T-cell lymphoma (HuT78) cells. 6-Methoxy-2-(trifluoromethyl)quinoline 5e and 6-methylcoumarin 6d, and their rhenium(I) tricarbonyl complexes 5eRe and 6dRe were found to arrest the cell cycle of HuT78 cells by causing a significant accumulation of cells in the G0/G1 phase and a marked decrease in the number of cells in the G2/M phase. These rhenium(I) tricarbonyl complexes also slightly increased ROS production and significantly decreased the mitochondrial membrane potential by 50 % (5eRe) and 45 % (6dRe) compared to untreated cells and cells treated with 5e and 6d. These results suggest that the cytotoxic effects of these compounds are mediated by their effects on mitochondrial membrane potential and the subsequent increase in ROS production.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Inorganic Biochemistry
生物-生化与分子生物学
CiteScore
7.00
自引率
10.30%
发文量
336
审稿时长
41 days
期刊介绍:
The Journal of Inorganic Biochemistry is an established international forum for research in all aspects of Biological Inorganic Chemistry. Original papers of a high scientific level are published in the form of Articles (full length papers), Short Communications, Focused Reviews and Bioinorganic Methods. Topics include: the chemistry, structure and function of metalloenzymes; the interaction of inorganic ions and molecules with proteins and nucleic acids; the synthesis and properties of coordination complexes of biological interest including both structural and functional model systems; the function of metal- containing systems in the regulation of gene expression; the role of metals in medicine; the application of spectroscopic methods to determine the structure of metallobiomolecules; the preparation and characterization of metal-based biomaterials; and related systems. The emphasis of the Journal is on the structure and mechanism of action of metallobiomolecules.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


