超共轭控制的分子构象可削弱锂离子溶解并稳定锂金属阳极
IF 7.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
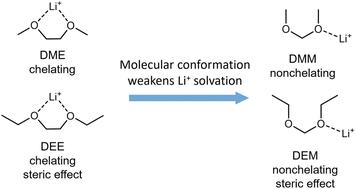
通过电解质工程调整锂离子的溶解结构已被证明对金属锂(Li)阳极有效。要绕过反复试验的做法,取得进一步的进展,有赖于建立分子设计原则。我们扩大了之前在溶剂氟化方面的工作范围,在此报告一种可降低成本、环境影响和毒性的无氟溶剂的替代设计原则。通过系统地研究无氟醚,我们发现短链乙缩醛由于超共轭作用而有利于[gauche, gauche]分子构象,从而导致与 Li+ 的单齿配位减弱。二甲氧基甲烷电解质的激活速度很快,库仑效率(CE)达到 99%,离子电导率高达 8.03 mS cm-1。在电流密度高达 4 mA cm-2 的无阳极 "铜 "LFP 袋式电池(70 至 100 次循环)和薄锂 "高负载 "LFP 纽扣电池(200 至 300 次循环)中,这种电解质的性能得到了验证。总之,与乙二醇醚相比,我们证明并合理解释了缩醛结构对锂金属循环性的改善。我们期待通过调整缩醛结构进一步提高性能。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Hyperconjugation-controlled molecular conformation weakens lithium-ion solvation and stabilizes lithium metal anodes
Tuning the solvation structure of lithium ions via electrolyte engineering has proven effective for lithium metal (Li) anodes. Further advancement that bypasses the trial-and-error practice relies on the establishment of molecular design principles. Expanding the scope of our previous work on solvent fluorination, we report here an alternative design principle for non-fluorinated solvents, which potentially have reduced cost, environmental impact, and toxicity. By studying non-fluorinated ethers systematically, we found that the short-chain acetals favor the [gauche, gauche] molecular conformation due to hyperconjugation, which leads to weakened monodentate coordination with Li+. The dimethoxymethane electrolyte showed fast activation to >99% coulombic efficiency (CE) and high ionic conductivity of 8.03 mS cm−1. The electrolyte performance was demonstrated in anode-free Cu‖LFP pouch cells at current densities up to 4 mA cm−2 (70 to 100 cycles) and thin-Li‖high-loading-LFP coin cells (200–300 cycles). Overall, we demonstrated and rationalized the improvement in Li metal cyclability by the acetal structure compared to ethylene glycol ethers. We expect further improvement in performance by tuning the acetal structure.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Science
CHEMISTRY, MULTIDISCIPLINARY-
CiteScore
14.40
自引率
4.80%
发文量
1352
审稿时长
2.1 months
期刊介绍:
Chemical Science is a journal that encompasses various disciplines within the chemical sciences. Its scope includes publishing ground-breaking research with significant implications for its respective field, as well as appealing to a wider audience in related areas. To be considered for publication, articles must showcase innovative and original advances in their field of study and be presented in a manner that is understandable to scientists from diverse backgrounds. However, the journal generally does not publish highly specialized research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


