探索铝-离子 (Al3+) 在钒铵青铜 (NH4V4O10) 中的嵌入,用于水性铝-离子充电电池
IF 3.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
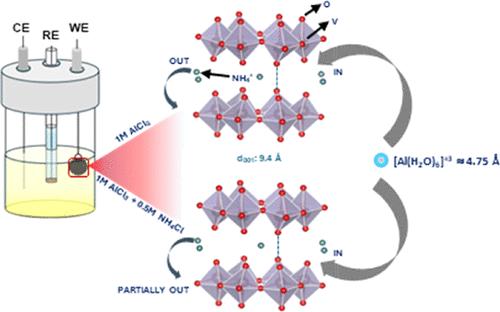
可充电铝离子电池(AIBs)因其具有竞争力的能量密度而有望成为锂电池的替代品。水性 AIB 可以使用具有开放式框架结构和大层间距的电极材料,这有利于铝离子的插入和萃取。在此,我们探讨了具有 9.4 Å 高层间距的钒酸铵(NH4V4O10;NVO)作为 AIBs 潜在正极的可能性。该材料在 1 M AlCl3 电解液中显示出 210 mA h g-1 的初始高放电容量,但由于插层[Al(H2O)6]3+ 阳离子引起的应变导致结构畸变而退化。研究了铵盐添加剂(NH4X:X = Cl、F、CH3CO2、HCO2)对电化学性能的影响,重点是 NH4Cl,结果表明其结构稳定性明显优于 1 M AlCl3。原位 XRD、傅立叶变换红外光谱、X 射线光电子能谱和电感耦合等离子体-光发射光谱分析表明,NVO 结构在几十次循环后部分稳定,循环能力增强。以 1 M AlCl3 为盐的溶剂成分调整也显示出类似的趋势。这项工作除了确定最佳的 Al3+ 插层宿主外,还强调了电解质在推进水性 AIB 技术中的关键作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Exploring Aluminum-Ion (Al3+) Insertion in Ammonium Vanadium Bronze (NH4V4O10) for Aqueous Aluminum-Ion Rechargeable Batteries
Rechargeable aluminum-ion batteries (AIBs) are promising alternatives to lithium-based batteries due to their competitive energy densities. Aqueous AIBs enable the use of electrode materials with open-framework structures and large interlayer spacings, which facilitate aluminum ion insertion and extraction. Herein, we explore ammonium vanadate (NH4V4O10; NVO) with a high interlayer spacing of ∼9.4 Å as a potential positive electrode for AIBs. The material demonstrates an initial high discharge capacity of 210 mA h g–1 in 1 M AlCl3 electrolyte but degrades due to structural distortion from the strain induced by the intercalating [Al(H2O)6]3+ cation. The effects of ammonium salt additives (NH4X: X = Cl, F, CH3CO2, HCO2) on the electrochemical performance are investigated, with a detailed focus on NH4Cl, demonstrating notable improvements in structural stability over 1 M AlCl3. Ex situ XRD, Fourier transform infrared, X-ray photoelectron spectroscopy, and inductively coupled plasma-optical emission spectrometry analyses reveal partial stabilization of the NVO structure and enhanced cyclability over a few tens of cycles. Solvent composition adjustments with 1 M AlCl3 as the salt showed similar trends. This work, in addition to identifying optimal Al3+ intercalating hosts, emphasizes the critical role of electrolytes in advancing aqueous AIB technologies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


