钌取代的多氧阴离子在乙烯到乙二醇的选择性电氧化过程中充当氧化还原梭子和中间稳定剂
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
当今乙二醇(EG)生产的高碳强度激发了人们对乙烯电氧化的兴趣。我们注意到之前关于小碳氢化合物有机电氧化的报道中存在动力学不良的问题,因此我们探索设计能激活并同时稳定轻烯的介质。一种钌取代的聚氧化金属盐(Ru-POM,{Si[Ru(H2O)W11O39]}5-)在环境条件下以 100 mA/cm2 的电流生产 EG 时,远红外效率达到 82%。通过原位光谱技术、电化学研究和密度泛函理论计算的结合,我们发现了两步氧化机制的证据:Ru-POM 首先经过电化学氧化进入高价态,通过部分氧化激活乙烯,形成中间复合物;然后,中间复合物迁移到阳极,在阳极进一步氧化生成 EG。Ru-POM 介导的电催化系统降低了 EG 生产所需的预计能耗,每吨 EG 只需 9 千兆焦耳(同时产生 0.04 吨 H2),而之前的典型工艺每吨需要 20-30 千兆焦耳。本文章由计算机程序翻译,如有差异,请以英文原文为准。
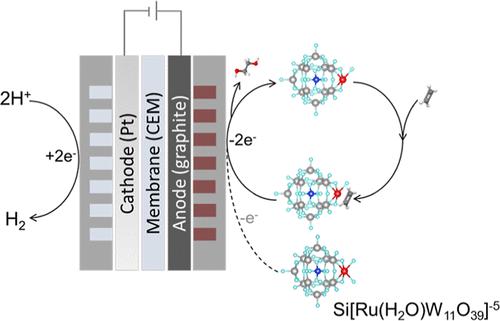
Ruthenium-Substituted Polyoxoanion Serves as Redox Shuttle and Intermediate Stabilizer in Selective Electrooxidation of Ethylene to Ethylene Glycol
The high carbon intensity of present-day ethylene glycol (EG) production motivates interest in electrifying ethylene oxidation. Noting poor kinetics in prior reports of the organic electrooxidation of small hydrocarbons, we explored the design of mediators that activate and simultaneously stabilize light alkenes. A ruthenium-substituted polyoxometalate (Ru-POM, {Si[Ru(H2O)W11O39]}5–) achieves 82% faradaic efficiency in EG production at 100 mA/cm2 under ambient conditions. Via the union of in situ spectroscopic techniques, electrochemical studies, and density functional theory calculations, we find evidence of a two-step oxidation mechanism: Ru-POM first undergoes electrochemical oxidation to the high valent state, activating ethylene via partial oxidation and forming an intermediate complex; this intermediate complex then migrates to the anode where it undergoes further oxidation to produce EG. The Ru-POM-mediated electrocatalytic system reduces the projected energy consumption required in EG production, requiring 9 GJ per ton of EG (and accompanied by 0.04 ton H2 coproduction), compared to 20–30 GJ/ton in typical prior processes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


