肝细胞中 Wars1 的下调会诱导线粒体应激并破坏代谢平衡。
IF 10.8
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
摘要
包括我们在内的一些实验室采用 Slc25a47tm1c(EUCOMM)Hmgu 小鼠模型来研究肝细胞特异性线粒体载体 SLC25A47 在调节肝脏代谢和全身生理学中的作用。在这项研究中,我们发现在肝细胞中重组 Slc25a47-Wars1 基因座后观察到的肝脏和全身表型主要是由位于 Slc25a47 附近的细胞色氨酸氨基酰-tRNA 合成酶 Wars1 的意外下调驱动的。虽然 Wars1 的下调会影响细胞膜翻译,但我们也观察到肝细胞内线粒体蛋白质合成受到了显著损害。线粒体功能的这种紊乱导致线粒体未折叠蛋白反应(UPRmt)的激活,而UPRmt是线粒体应激反应(MSR)的一个重要组成部分。我们的研究结果阐明了 Slc25a47 和 Wars1 在维持全身和肝脏代谢平衡中的不同作用。这项研究揭示了氨基酰-tRNA 合成酶在线粒体生理和应激反应中的广泛意义。本文章由计算机程序翻译,如有差异,请以英文原文为准。
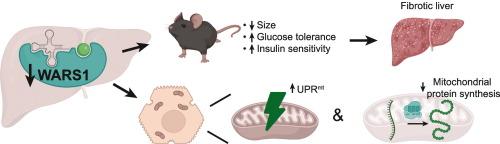
Wars1 downregulation in hepatocytes induces mitochondrial stress and disrupts metabolic homeostasis
Several laboratories, including ours, have employed the Slc25a47tm1c(EUCOMM)Hmgu mouse model to investigate the role of SLC25A47, a hepatocyte-specific mitochondrial carrier, in regulating hepatic metabolism and systemic physiology. In this study, we reveal that the hepatic and systemic phenotypes observed following recombination of the Slc25a47-Wars1 locus in hepatocytes are primarily driven by the unexpected downregulation of Wars1, the cytosolic tryptophan aminoacyl-tRNA synthetase located adjacent to Slc25a47. While the downregulation of Wars1 predictably affects cytosolic translation, we also observed a significant impairment in mitochondrial protein synthesis within hepatocytes. This disturbance in mitochondrial function leads to an activation of the mitochondrial unfolded protein response (UPRmt), a critical component of the mitochondrial stress response (MSR). Our findings clarify the distinct roles of Slc25a47 and Wars1 in maintaining both systemic and hepatic metabolic homeostasis. This study sheds new light on the broader implications of aminoacyl-tRNA synthetases in mitochondrial physiology and stress responses.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Metabolism: clinical and experimental
医学-内分泌学与代谢
CiteScore
18.90
自引率
3.10%
发文量
310
审稿时长
16 days
期刊介绍:
Metabolism upholds research excellence by disseminating high-quality original research, reviews, editorials, and commentaries covering all facets of human metabolism.
Consideration for publication in Metabolism extends to studies in humans, animal, and cellular models, with a particular emphasis on work demonstrating strong translational potential.
The journal addresses a range of topics, including:
- Energy Expenditure and Obesity
- Metabolic Syndrome, Prediabetes, and Diabetes
- Nutrition, Exercise, and the Environment
- Genetics and Genomics, Proteomics, and Metabolomics
- Carbohydrate, Lipid, and Protein Metabolism
- Endocrinology and Hypertension
- Mineral and Bone Metabolism
- Cardiovascular Diseases and Malignancies
- Inflammation in metabolism and immunometabolism
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


