胶质母细胞瘤中辐射诱导的衰老:机制和根除策略概述。
IF 5.2
2区 医学
Q1 MEDICINE, RESEARCH & EXPERIMENTAL
引用次数: 0
摘要
放疗作为胶质母细胞瘤的一种治疗方法,由于肿瘤内在的抗凋亡机制而受到限制。较高的放射剂量会导致正常组织和器官(如视神经脊索)中毒。细胞衰老是胶质母细胞瘤放疗后细胞凋亡的另一种机制,在正常细胞和肿瘤细胞中都会发生。虽然细胞衰老会阻碍肿瘤生长并维持细胞周期,但它也可能成为肿瘤发展和治疗后复发的原因。在这篇综述中,我们将详细探讨放射诱导的衰老在胶质母细胞瘤中的意义以及导致放射抗性的潜在机制。我们还讨论了衰老生物标志物和衰老相关分泌表型(SASP)在肿瘤复发中的作用。最后,我们回顾了对放射治疗后消除或抑制胶质母细胞瘤衰老细胞的潜在干预措施的研究。本文章由计算机程序翻译,如有差异,请以英文原文为准。
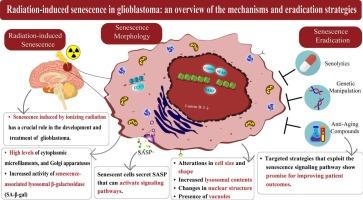
Radiation-induced senescence in glioblastoma: An overview of the mechanisms and eradication strategies
Radiotherapy as a treatment method for glioblastoma is limited due to the intrinsic apoptosis resistance mechanisms of the tumor. Administration of higher radiation doses contributes to toxicities in normal tissues and organs at risk, like optic chiasma. Cellular senescence represents an alternative mechanism to apoptosis following radiotherapy in glioblastoma, occurring in both normal and neoplastic cells. Although it impedes the growth of tumors and sustains cells in their cycle, it can also act as a cause of tumor development and recurrence following treatment. In this review, we discuss detailed insights into the significance of radiation-induced senescence in glioblastoma and the underlying mechanisms that lead to radioresistance. We also discuss senescence biomarkers and the role of senescence-associated secretory phenotype (SASP) in tumor recurrence. Finally, we review the studies that have administered potential interventions to eradicate or inhibit senescent cells in glioblastoma after treatment with radiation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Life sciences
医学-药学
CiteScore
12.20
自引率
1.60%
发文量
841
审稿时长
6 months
期刊介绍:
Life Sciences is an international journal publishing articles that emphasize the molecular, cellular, and functional basis of therapy. The journal emphasizes the understanding of mechanism that is relevant to all aspects of human disease and translation to patients. All articles are rigorously reviewed.
The Journal favors publication of full-length papers where modern scientific technologies are used to explain molecular, cellular and physiological mechanisms. Articles that merely report observations are rarely accepted. Recommendations from the Declaration of Helsinki or NIH guidelines for care and use of laboratory animals must be adhered to. Articles should be written at a level accessible to readers who are non-specialists in the topic of the article themselves, but who are interested in the research. The Journal welcomes reviews on topics of wide interest to investigators in the life sciences. We particularly encourage submission of brief, focused reviews containing high-quality artwork and require the use of mechanistic summary diagrams.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


