沉积在还原 TiO2(110) 上的 Pt13 纳米团簇上与形状有关的 CO 化学吸附。
IF 2.7
4区 生物学
Q2 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
摘要
本文深入研究了纳米颗粒形状对一氧化碳化学吸附的影响,以及支撑在还原 TiO2(110) 上的 Pt13 纳米催化剂的反应活性。纳米颗粒均由 13 个铂原子组成,但形状各异,它们在吸附一氧化碳时的反应性也不尽相同。计算得出的形成能和一氧化碳吸附能与系统的电子特性和相关铂原子的氧化态相关。通过分析铂簇沉积到氧化物上过程中的能带偏移,以及比较价带最大值与 CO 到 CO2 反应中测得的氧化电位,我们预测了该体系在该反应中的氧化能力。我们的研究结果表明,具有立方体、双三角形 DT2 和八面体 Oh 形状的 Pt13 簇拥在表面上,特别有利于催化 CO 到 CO2 的转化。在这些几何形状中,对几种吸附 CO 的构型进行了评估,重点是阴离子铂位点和阳离子铂位点之间的比例。这一比例似乎决定了 CO 氧化的活性,这与最近的实验报告一致。本文章由计算机程序翻译,如有差异,请以英文原文为准。
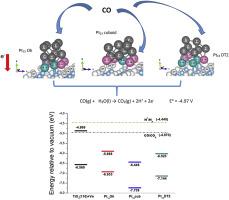
Shape-dependent CO chemisorption on Pt13 nanocluster deposited on reduced TiO2(110)
This article delves into the impact of nanoparticle shape on CO chemisorption and the reactivity of Pt13 nanocatalysts supported on reduced TiO2(110). Distinct reactivity in carbon monoxide adsorption is observed among nanoparticles, all composed of 13 platinum atoms but varying in shape. The calculated formation and CO adsorption energies are correlated to the electronic properties of the system and the oxidation states of the Pt atoms involved. Through an analysis of band shifting during the deposition of Pt clusters onto the oxide, and a comparison of the valence band maximum with the measured oxidation potential for the CO to CO2 reaction, we make predictions about the system's oxidation capability in this reaction. Our findings suggest that Pt13 clusters with cuboid, double triangle DT2 and octahedral Oh shapes, supported on the surface, are particularly advantageous for catalyzing the conversion of CO to CO2. Within these geometries, several configurations for CO adsorption are evaluated, focusing on the ratio between anionic and cationic Pt sites. This ratio appears to govern the activity for CO oxidation, aligning with recent experimental reports.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of molecular graphics & modelling
生物-计算机:跨学科应用
CiteScore
5.50
自引率
6.90%
发文量
216
审稿时长
35 days
期刊介绍:
The Journal of Molecular Graphics and Modelling is devoted to the publication of papers on the uses of computers in theoretical investigations of molecular structure, function, interaction, and design. The scope of the journal includes all aspects of molecular modeling and computational chemistry, including, for instance, the study of molecular shape and properties, molecular simulations, protein and polymer engineering, drug design, materials design, structure-activity and structure-property relationships, database mining, and compound library design.
As a primary research journal, JMGM seeks to bring new knowledge to the attention of our readers. As such, submissions to the journal need to not only report results, but must draw conclusions and explore implications of the work presented. Authors are strongly encouraged to bear this in mind when preparing manuscripts. Routine applications of standard modelling approaches, providing only very limited new scientific insight, will not meet our criteria for publication. Reproducibility of reported calculations is an important issue. Wherever possible, we urge authors to enhance their papers with Supplementary Data, for example, in QSAR studies machine-readable versions of molecular datasets or in the development of new force-field parameters versions of the topology and force field parameter files. Routine applications of existing methods that do not lead to genuinely new insight will not be considered.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


