AXL/GAS6 信号调节乳腺癌中肿瘤相关巨噬细胞的分化。
IF 3.3
3区 生物学
Q3 CELL BIOLOGY
引用次数: 0
摘要
大多数上皮癌都有预后相关的骨髓单核细胞浸润。免疫抑制性肿瘤相关巨噬细胞(TAMs)及其前体单核细胞-MDSCs曾与人类乳腺癌(BCa)的不良预后相关,但免疫抑制性TAMs从髓单核细胞前体极化的机制尚未完全明了。在这项研究中,我们发现AXL/GAS6通路会改变巨噬细胞的表型,使其从HLA-DRhighCD206-low CD163-low经典吞噬型转变为HLA-DR-lowCD206-highCD163-high免疫抑制型,从而加速BCa的进展,并增加肿瘤床的血管生成特征和癌细胞的侵袭能力。值得注意的是,AXL和GAS6在人类浸润性乳腺癌中均表达上调,在三阴性组织学类型中表达最高。从机理上讲,我们证明了 AXL/GAS6 信号通过调节免疫抑制 IL10 的产生,同时抑制 BCa 肿瘤微环境(TME)中 IL-1β 的表达,从而驱动免疫抑制。此外,AXL/GAS6 信号通过激活 PI3K/AKT 和 NF-κB 信号通路促进血管生成。我们的研究结果揭示了AXL/GAS6轴在TAMs分化过程中的作用,TAMs分化决定着恶性肿瘤的生长,这表明解除AXL/GAS6轴的疗法可能会给原本无法治愈的三阴性乳腺癌(TNBC)患者带来治疗机会。本文章由计算机程序翻译,如有差异,请以英文原文为准。
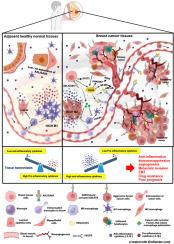
AXL/GAS6 signaling governs differentiation of tumor-associated macrophages in breast cancer
Most epithelial cancers are infiltrated by prognostically relevant myelomonocytic cells. Immunosuppressive tumor associated macrophages (TAMs) and their precursor monocytic myeloid-derived suppressor cells (MDSCs) have previously been associated with worse outcomes in human breast cancer (BCa), yet the mechanism of immunosuppressive TAMs-polarization from myelomonocytic precursors is not completely understood. In this study, we show that persuaded AXL/GAS6 pathway alters macrophage phenotype from HLA-DRhighCD206lowCD163low classical phagocytic into HLA-DRlowCD206highCD163high immunosuppressive ones with accelerated BCa progression, and increased angiogenesis signature and invasion ability of cancer cells at tumor beds. Notably, both AXL and GAS6 expressions are upregulated in human invasive breast carcinoma, with maximum expression in triple negative histology type. Mechanistically, we demonstrate that AXL/GAS6 signaling drives immunosuppression by governing increased immunosuppressive IL10 production while dampening IL-1β expression within the tumor microenvironment (TME) of BCa. Further, AXL/GAS6 signaling promotes angiogenesis through the activation of PI3K/AKT and NF-κB signaling pathways. Our results unveil role of AXL/GAS6 axis in the differentiation of TAMs, which governs malignant growth, and suggest that therapies that uncouple AXL/GAS6 axis may exhibit therapeutic opportunity for otherwise undruggable Triple Negative Breast Cancer (TNBC) patients.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Experimental cell research
医学-细胞生物学
CiteScore
7.20
自引率
0.00%
发文量
295
审稿时长
30 days
期刊介绍:
Our scope includes but is not limited to areas such as: Chromosome biology; Chromatin and epigenetics; DNA repair; Gene regulation; Nuclear import-export; RNA processing; Non-coding RNAs; Organelle biology; The cytoskeleton; Intracellular trafficking; Cell-cell and cell-matrix interactions; Cell motility and migration; Cell proliferation; Cellular differentiation; Signal transduction; Programmed cell death.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


