萘丁通过抑制过氧化物酶体增殖激活受体γ(PPARG)改善肥胖/肥胖小鼠的肝脏脂肪变性。
IF 5.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
非酒精性脂肪肝(NAFLD)是最主要的代谢性肝病,目前缺乏有效、安全的药物干预措施。水飞蓟素(DA)是一种天然香豆素衍生物,具有抗炎和抗氧化活性,是一种治疗非酒精性脂肪肝的有前途的药物。在这项研究中,我们评估了DA对肥胖/肥胖小鼠肝脂代谢的影响和机制。结果表明,DA能有效改善肥胖/ob小鼠的糖代谢和肝脏脂质积累。代谢组学和 RNA 测序(RNA-seq)结合 GEO 数据分析表明,DA 主要调节过氧化物酶体增殖激活受体γ(PPARG)通路,这在肥胖/肥胖小鼠体内得到了验证。从机理上讲,DA 通过抑制 PPARG 启动子的活性并下调其表达,选择性地靶向肝细胞中的 PPARG,导致下游脂质代谢相关基因(包括脂肪酸结合蛋白 4(Fabp4)、分化簇 36(Cd36)和脂肪酸合成酶(Fasn))的转录减少。这种效应在棕榈酸(PA)损伤的 PPARG 缺陷 HepG2 细胞中被取消。我们的研究结果提供了 DA 可选择性抑制肝 PPARG 的证据,这表明通过靶向 PPARG 来预防肝脂肪变性是一种有吸引力的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
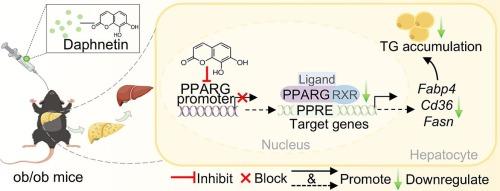
Daphnetin ameliorates hepatic steatosis by suppressing peroxisome proliferator-activated receptor gamma (PPARG) in ob/ob mice
Non-alcoholic fatty liver disease (NAFLD) is the predominant metabolic liver disorder and currently lacks effective and safe pharmaceutical interventions. Daphnetin (DA), a natural coumarin derivative with anti-inflammatory and antioxidant activities, is a promising agent for NAFLD treatment. In this study, we evaluated the effects and mechanisms of DA on hepatic lipid metabolism in ob/ob mice. Our results showed that DA effectively ameliorates glucose metabolism and hepatic lipid accumulation in ob/ob mice. Metabolomics and RNA sequencing (RNA-seq), combined with GEO data analysis, suggest that DA primarily modulates the peroxisome proliferator-activated receptor gamma (PPARG) pathway, as validated in vivo in ob/ob mice. Mechanistically, DA selectively targets PPARG in hepatic cells by inhibiting PPARG promoter activity and downregulating its expression, resulting in decreased transcription of downstream lipid metabolism-related genes, including fatty acid binding protein 4 (Fabp4), cluster of differentiation 36 (Cd36), and fatty acid synthase (Fasn). This effect was abolished in PPARG-deficient HepG2 cells subjected to palmitic acid (PA) insult. Our findings provide evidence that DA acts as a selective suppressor of hepatic PPARG, suggesting an attractive strategy by targeting PPARG for the prevention of hepatic steatosis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biochemical pharmacology
医学-药学
CiteScore
10.30
自引率
1.70%
发文量
420
审稿时长
17 days
期刊介绍:
Biochemical Pharmacology publishes original research findings, Commentaries and review articles related to the elucidation of cellular and tissue function(s) at the biochemical and molecular levels, the modification of cellular phenotype(s) by genetic, transcriptional/translational or drug/compound-induced modifications, as well as the pharmacodynamics and pharmacokinetics of xenobiotics and drugs, the latter including both small molecules and biologics.
The journal''s target audience includes scientists engaged in the identification and study of the mechanisms of action of xenobiotics, biologics and drugs and in the drug discovery and development process.
All areas of cellular biology and cellular, tissue/organ and whole animal pharmacology fall within the scope of the journal. Drug classes covered include anti-infectives, anti-inflammatory agents, chemotherapeutics, cardiovascular, endocrinological, immunological, metabolic, neurological and psychiatric drugs, as well as research on drug metabolism and kinetics. While medicinal chemistry is a topic of complimentary interest, manuscripts in this area must contain sufficient biological data to characterize pharmacologically the compounds reported. Submissions describing work focused predominately on chemical synthesis and molecular modeling will not be considered for review.
While particular emphasis is placed on reporting the results of molecular and biochemical studies, research involving the use of tissue and animal models of human pathophysiology and toxicology is of interest to the extent that it helps define drug mechanisms of action, safety and efficacy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


