通过共价δ键与晚期镧系元素稳定的四阴离子苯线性反三明治配合物
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
报告了一系列 (CpiPr5Ln)2(μ-η6:η6-C6H6)(1-Ln)(Ln = Y、Gd、Tb、Dy、Tm)类型的二镧系苯反夹心配合物。这些化合物是在苯的存在下,通过在二乙醚中用石墨钾还原各自的三价二聚体 CpiPr52Ln2I4(Ln = Y、Gd、Tb、Dy、Tm)而合成的。1-Ln 的 Ln-Bzcentroid 距离是迄今为止观察到的最短距离,从 1-Tm 的 1.943(1) Å 到 1-Gd 的 2.039(6) Å 不等。结构、光谱和磁性分析以及密度泛函理论计算都支持每个化合物中都存在一种罕见的未取代的四阴离子苯,这种苯通过涉及 (C6H6)4- 的填充 π* 轨道和 Ln3+ 离子的空闲 dxy 和 dx2-y2 轨道的强δ共价键相互作用而得到稳定。值得注意的是,1-Ln 是后期镧系元素化合物中第一个以未取代的四阴离子苯为特征的例子。本文章由计算机程序翻译,如有差异,请以英文原文为准。
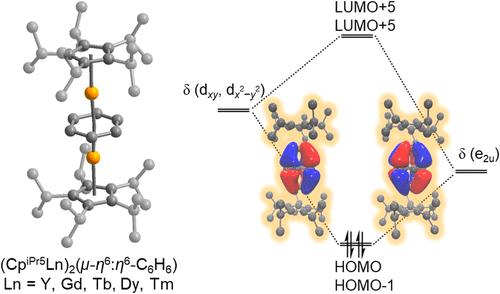
Linear Inverse Sandwich Complexes of Tetraanionic Benzene Stabilized by Covalent δ-Bonding with Late Lanthanides
A series of dilanthanide benzene inverse sandwich complexes of the type (CpiPr5Ln)2(μ–η6:η6-C6H6) (1-Ln) (Ln = Y, Gd, Tb, Dy, Tm) are reported. These compounds are synthesized by reduction of the respective trivalent dimers CpiPr52Ln2I4 (Ln = Y, Gd, Tb, Dy, Tm) in diethyl ether with potassium graphite in the presence of benzene, and they feature an unusual linear coordination geometry with a highly planar benzene bridge as verified by single-crystal X-ray diffraction. The Ln–Bzcentroid distances of 1-Ln are the shortest distances observed to date, ranging from 1.943(1) Å for 1-Tm to 2.039(6) Å for 1-Gd. Structural, spectroscopic, and magnetic analyses together with density functional theory calculations support the presence of a rare, unsubstituted tetraanionic benzene in each compound, which is stabilized by strong covalent δ bonding interactions involving the filled π* orbitals of (C6H6)4– and vacant dxy and dx2–y2 orbitals of the Ln3+ ions. Notably, 1-Ln are the first examples of compounds of the later lanthanides to feature an unsubstituted tetraanionic benzene.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


