界面工程化 CuxO@Bi2MoO6 异质结抑制压电筛选效应并促进抗菌治疗的双纳米酶催化作用
IF 4.1
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
声动力疗法面临着声敏化剂声效低的问题,而纳米酶又具有内在的低催化活性。在此,为了提高 N 型压电半导体的压电势,设计了 P-N 异质结来抑制压电筛选效应(PSE),提高电子利用效率,从而增强纳米酶的活性。在 N 型压电 Bi2MoO6(BMO)纳米片(NFs)上原位生长 P 型 CuxO 纳米粒子,通过界面工程构建异质结构 CuxO@BMO。CuxO 沉积会导致 BMO NF 的晶格畸变,从而改善压电响应,而强界面电场 (IEF) 则会抑制 PSE 并增加压电势。非局部压电势、局部 IEF 和谷胱甘肽(GSH)的接种增强了电子-空穴分离,并以高选择性提高了 BMO 的过氧化物酶(POD)样活性和 CuxO 的 GSH 氧化酶(GSHOx)样活性。异质结的形成导致了界面电子的转移和重排,压电势的增加加速了与细菌界面的电子转移,从而增加了活性氧化物的产生,干扰了三磷酸腺苷的合成。异质结构纳米酶产生大量细胞内-OH,使存活细菌减少 4log 数量级,并有效分散生物膜。这项研究阐明了纳米酶和声催化的整体机制,为高压电势和纳米酶催化疗法的协同作用开辟了一条新途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
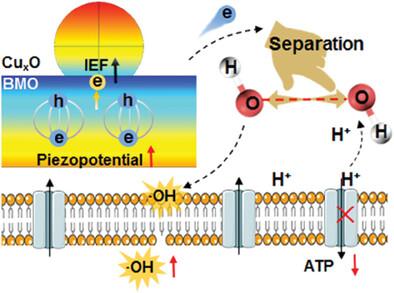
Interface-Engineered CuxO@Bi2MoO6 Heterojunctions to Inhibit Piezoelectric Screening Effect and Promote Double-Nanozyme Catalysis for Antibacterial Treatment
Sonodynamic therapy is confronted with the low acoustic efficiency of sonosensitizers, and nanozymes are accompanied by intrinsic low catalytic activity. Herein, to increase the piezopotential of N-type piezoelectric semiconductors, the P-N heterojunction is designed to inhibit the piezoelectric screening effect (PSE) and increase electron utilization efficiency to enhance nanozyme activity. P-type CuxO nanoparticles are in situ grown on N-type piezoelectric Bi2MoO6 (BMO) nanoflakes (NFs) to construct heterostructured CuxO@BMO by interface engineering. CuxO deposition leads to lattice distortion of BMO NFs to improve piezoelectric response, and the strong interface electric field (IEF) suppresses PSE and increases piezopotential. The nonlocal piezopotential, local IEF, and glutathione (GSH) inoculation enhances electron−hole separation and increases peroxidase (POD)-like activity of BMO and GSH oxidase (GSHOx)-like activity of CuxO with high selectivity. The heterojunction formation causes the transfer and rearrangement of interface electrons, and the increased piezopotential accelerates electron transfer at interfaces with bacteria, thus increasing the production of reactive oxidative species and interfering with adenosine triphosphate synthesis. The heterostructured nanozymes produce abundant intracellular ·OH and achieve 4log magnitude reductions in viable bacteria and effective biofilm dispersion. This study elucidates integral mechanisms of nanozyme and acoustic catalysis and opens up a new way to synergize high piezopotential and nanozyme-catalyzed therapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Chemical Neuroscience
BIOCHEMISTRY & MOLECULAR BIOLOGY-CHEMISTRY, MEDICINAL
CiteScore
9.20
自引率
4.00%
发文量
323
审稿时长
1 months
期刊介绍:
ACS Chemical Neuroscience publishes high-quality research articles and reviews that showcase chemical, quantitative biological, biophysical and bioengineering approaches to the understanding of the nervous system and to the development of new treatments for neurological disorders. Research in the journal focuses on aspects of chemical neurobiology and bio-neurochemistry such as the following:
Neurotransmitters and receptors
Neuropharmaceuticals and therapeutics
Neural development—Plasticity, and degeneration
Chemical, physical, and computational methods in neuroscience
Neuronal diseases—basis, detection, and treatment
Mechanism of aging, learning, memory and behavior
Pain and sensory processing
Neurotoxins
Neuroscience-inspired bioengineering
Development of methods in chemical neurobiology
Neuroimaging agents and technologies
Animal models for central nervous system diseases
Behavioral research
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


