改变 Ni/Al2O3 催化剂的特性以提高催化氨分解的绿色制氢能力:pH 值确实很重要!
IF 4.1
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
本研究首次研究了阳离子-阴离子双水解(CADH)法形成 Ni/Al2O3 催化剂的溶液 pH 值(6.0-12.5)对绿色制氢 NH3 分解催化性能的影响。通过各种分析技术对所制备催化剂的理化性质进行了系统表征。结果表明,pH 条件对所制备的 Ni/Al2O3 催化剂的结构特性和催化活性均有显著影响。在高 pH 条件(pH ≥ 10.0)下制备的催化剂对 NH3 分解具有更好的催化活性,这主要是由于 Ni 与 Al2O3 载体之间的相互作用具有适当的协同效应,Ni 具有较大的活性表面积,以及催化剂具有合适的孔隙率、粒度和碱性。相关分析和密度泛函理论(DFT)计算证实,表面金属镍的比例对控制镍/Al2O3 催化剂的催化活性起着至关重要的作用。在 600 °C、NH3 WHSV 为 54000 mL/gcat./h 的苛刻反应条件下,40Ni/Al2O3 催化剂(Ni = 40 wt%)的 NH3 转化率超过 94.5%,并能稳定维持 200 小时而无明显失活。总之,我们的工作不仅强调了在 CADH 溶液中 pH 条件对于经济高效地制备 Ni/Al2O3 催化剂的关键作用,而且还为开发一种高活性、稳定且不含 Ru 的催化剂提出了一种前景广阔的策略,可用于通过 NH3 分解进行实用制氢。本文章由计算机程序翻译,如有差异,请以英文原文为准。
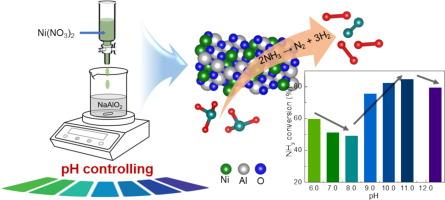
Turning properties of Ni/Al2O3 catalyst to improve catalytic ammonia decomposition for green hydrogen production: pH does matter!
In this study, the influence of solution pH (6.0–12.5) of the cation–anion double hydrolysis (CADH) method in the formation of Ni/Al2O3 catalyst and its catalytic performance for green hydrogen production NH3 decomposition was studied for the first time. The physicochemical properties of the prepared catalysts were systematically characterized by various analysis techniques. The results indicated that pH conditions significantly influenced both the structural properties and catalytic activity of the derived Ni/Al2O3 catalysts. The better catalytic activity for NH3 decomposition over catalysts prepared under high pH conditions (pH ≥ 10.0) is mainly due to the appropriate synergic effect of the interaction between Ni and Al2O3 support, large active Ni surface area, and suitable porosity, particle size, and basicity of the catalyst. The correlation analysis and density functional theory (DFT) calculations confirm that the percentage of surface metallic Ni plays a crucial role in controlling the catalytic activity of Ni/Al2O3 catalysts. The 40Ni/Al2O3 catalyst (Ni = 40 wt%) could achieve over 94.5 % NH3 conversion under the harsh reaction conditions of 600 °C and NH3 WHSV of 54000 mL/gcat./h, and that stably maintained for 200 h without any obvious deactivation. Overall, our work not only highlights the critical role of pH conditions in the CADH solution for cost-effective Ni/Al2O3 catalyst preparation but also proposes a promising strategy for developing a highly active, stable, and Ru-free catalyst for practical hydrogen production via NH3 decomposition.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Chemical Neuroscience
BIOCHEMISTRY & MOLECULAR BIOLOGY-CHEMISTRY, MEDICINAL
CiteScore
9.20
自引率
4.00%
发文量
323
审稿时长
1 months
期刊介绍:
ACS Chemical Neuroscience publishes high-quality research articles and reviews that showcase chemical, quantitative biological, biophysical and bioengineering approaches to the understanding of the nervous system and to the development of new treatments for neurological disorders. Research in the journal focuses on aspects of chemical neurobiology and bio-neurochemistry such as the following:
Neurotransmitters and receptors
Neuropharmaceuticals and therapeutics
Neural development—Plasticity, and degeneration
Chemical, physical, and computational methods in neuroscience
Neuronal diseases—basis, detection, and treatment
Mechanism of aging, learning, memory and behavior
Pain and sensory processing
Neurotoxins
Neuroscience-inspired bioengineering
Development of methods in chemical neurobiology
Neuroimaging agents and technologies
Animal models for central nervous system diseases
Behavioral research
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


