调节氧化途径机制,实现可持续酸性水氧化
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
发展酸稳定性氧进化反应(OER)电催化剂对于通过质子交换膜(PEM)水电解法高效制氢至关重要。遗憾的是,电催化剂的活性受制于吸附进化机制中的线性比例关系,而晶格氧介导机制则破坏了其稳定性。在此,我们提出了一种异质双位点氧化物通路机制(OPM),通过直接二氧自由基耦合避免了这些限制。路易斯酸(Cr)和 Ru 结合形成固溶体氧化物(CrxRu1-xO2)可促进 OH 的吸附并缩短双位点距离,这有利于 *O 自由基的形成并促进二氧自由基的耦合,从而将 OER 机制改变为 Cr-Ru 双位点 OPM。与 RuO2 相比,Cr0.6Ru0.4O2 催化剂的过电位更低,并能在 300 mA cm-2 的 PEM 水电解槽中稳定运行 350 小时以上。这种机理调节策略为大规模绿色制氢所必需的最佳催化途径铺平了道路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
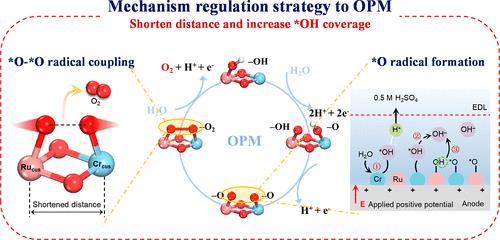
Regulation of Oxide Pathway Mechanism for Sustainable Acidic Water Oxidation
The advancement of acid-stable oxygen evolution reaction (OER) electrocatalysts is crucial for efficient hydrogen production through proton exchange membrane (PEM) water electrolysis. Unfortunately, the activity of electrocatalysts is constrained by a linear scaling relationship in the adsorbed evolution mechanism, while the lattice-oxygen-mediated mechanism undermines stability. Here, we propose a heterogeneous dual-site oxide pathway mechanism (OPM) that avoids these limitations through direct dioxygen radical coupling. A combination of Lewis acid (Cr) and Ru to form solid solution oxides (CrxRu1–xO2) promotes OH adsorption and shortens the dual-site distance, which facilitates the formation of *O radical and promotes the coupling of dioxygen radical, thereby altering the OER mechanism to a Cr–Ru dual-site OPM. The Cr0.6Ru0.4O2 catalyst demonstrates a lower overpotential than that of RuO2 and maintains stable operation for over 350 h in a PEM water electrolyzer at 300 mA cm–2. This mechanism regulation strategy paves the way for an optimal catalytic pathway, essential for large-scale green hydrogen production.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


