钴-氢化物催化的烯丙基羧酸酯转位 (ACT)
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
烯丙基羧酸酯的烯丙基-羧酸酯转位(ACT)是有机化学中原子经济性和合成可靠性最高的转化之一,因为烯丙基羧酸酯是用途广泛的合成中间体。经典的烯丙基羧酸酯转化(包括[3,3]-对位重排和过渡金属催化的烯丙基重排)通常通过双电子过程产生 1,2-烯/1,3-乙酰氧基变换产物。然而,通过改变 ACT 的位置来产生不同的 1,3-烯/1,2-乙酰氧基位移产物的方法仍未出现。在此,我们首次报道了钴酸酐催化的烯丙基羧酸酯 ACT,通过 1,2-radical migration (RaM) 策略获得了这些前所未有的 1,3-alkene/1,2-acyloxy shifted 产物。这种转化具有广泛的官能团耐受性,适用于复杂分子的后期改性,适合克级合成。它还扩展了烯丙基羧酸酯和钴催化的反应范围。初步实验和计算研究表明,其机理涉及金属-氢化物氢原子转移(MHAT)和 1,2-RaM 过程。该反应有望成为开发烯丙基羧酸酯多功能 Co-H 催化转化的基础,为合成、医药和材料化学提供大量有价值的构筑基块。本文章由计算机程序翻译,如有差异,请以英文原文为准。
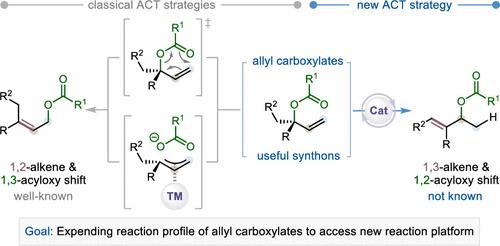
Cobalt-Hydride-Catalyzed Alkene-Carboxylate Transposition (ACT) of Allyl Carboxylates
The alkene-carboxylate transposition (ACT) of allyl carboxylates is one of the most atom-economic and synthetically reliable transformations in organic chemistry, as allyl carboxylates are versatile synthetic intermediates. Classic ACT transformations, including [3,3]-sigmatropic rearrangement and transition metal-catalyzed allylic rearrangement, typically yield 1,2-alkene/1,3-acyloxy shifted products through a two-electron process. However, position-altered ACT to produce distinct 1,3-alkene/1,2-acyloxy shifted products remains elusive. Here, we report the first cobalt-hydride-catalyzed ACT of allyl carboxylates, enabling access to these unprecedented 1,3-alkene/1,2-acyloxy shifted products via a 1,2-radical migration (RaM) strategy. This transformation demonstrates broad functional group tolerance, is suitable for late-stage modification of complex molecules, and is amenable to gram-scale synthesis. It also expands the reaction profiles of both allyl carboxylates and cobalt catalysis. Preliminary experimental and computational studies suggest a mechanism involving metal-hydride hydrogen atom transfer (MHAT) and the 1,2-RaM process. This reaction is expected to serve as the basis for the development of versatile Co–H-catalyzed transformations of allyl carboxylates, generating a wide array of valuable building blocks for synthetic, medicinal, and materials chemistry.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


