芳香环嵌合六氢环五呋喃与各种醛的化学歧化反应
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
与富含电子的芳香环融合的六氢-2H-环戊并[b]呋喃与各种芳香醛以不同的方式发生反应,从而构建出各种框架。与二甲氧基苯融合的环五呋喃反应生成与呋喃烃环融合的二奎烷、茚并吡喃或二芳基茚并烷,这取决于芳香醛的类型,而吲哚烷化的环五呋喃则生成与呋喃烃环融合的另一种二奎烷或亚苄基环五呋喃。这种化学差异是由于吲哚和二甲氧基苯融合的环戊并呋喃具有不同的性质。也就是说,路易斯酸促进底物的呋喃环打开后,会分别形成亲电或亲核中间体。此外,观察到的化学差异可归因于三种分类芳香醛的独特电子特性。特别值得注意的是,2,4-二甲氧基苯甲醛与二甲氧基苯融合的环五呋喃在苯环上发生反应,而与吲哚融合的环五呋喃则在甲酰基上发生反应。本文章由计算机程序翻译,如有差异,请以英文原文为准。
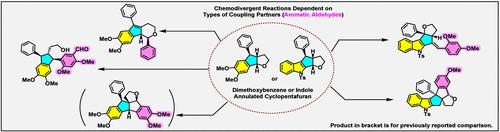
Chemodivergent Reactions of Aromatic Ring-Annulated Hexahydrocyclopentafurans with Various Aldehydes
Hexahydro-2H-cyclopenta[b]furan fused to electron-rich aromatic rings reacts with various aromatic aldehydes in different modes to build diverse frameworks. The reaction of a dimethoxybenzene-fused cyclopentafuran generated diquinanes fused with a hydrofuran ring, indenopyrans, or diarylindanes depending upon the type of aromatic aldehydes, whereas an indole-annulated cyclopentafuran generated another type of diquinane fused with hydrofuran ring or benzylidenecyclopentafuran. The chemodivergence is due to the different properties between indole- and dimethoxybenzene-fused hydrocyclopentafurans. Namely, Lewis acid-promoted furan-ring opening of the substrates resulted in the formation of an electrophilic or a nucleophilic intermediate, respectively. Additionally, the observed chemodivergence can be attributed to the distinctive electronic properties of three classified aromatic aldehydes. Of particular interest is that 2,4-dimethoxybenzaldehyde reacted with the dimethoxybenzene-fused cyclopentafuran at the benzene ring, whereas it reacted with the indole-fused cyclopentafuran at the formyl group.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


