铟镓锌氧化物:络合剂对结构的影响
IF 1.2
4区 化学
Q4 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
In2O3-Ga2O3-ZnO 三元氧化物具有电子应用前景。制造基于 In2O3-Ga2O3-ZnO 的晶体管的可行方法需要一种制备 X 射线纯粉末的技术。本文报告了所研究的有机络合剂对所获粉末形态影响的数据。结果表明,使用乙二醇比使用柠檬酸更可取,因为前者制备的粉末不含混合物相。本文章由计算机程序翻译,如有差异,请以英文原文为准。
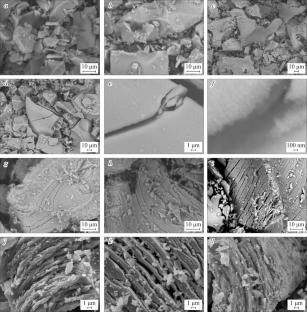
Indium–Gallium–Zinc Oxide: Influence of the Complexing Agent on the Structure
The In2O3–Ga2O3–ZnO ternary oxide is prospective for electronics applications. Promising methods of fabricating In2O3–Ga2O3–ZnO based transistors require a technique for the preparation of X-ray pure powders. Data on the influence of the studied organic complexing agent on the morphology of obtained powders are reported. It is shown that ethylene glycol is more preferable to use than citric acid since the powders prepared in the first case contain no admixture phases.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Structural Chemistry
化学-无机化学与核化学
CiteScore
1.60
自引率
12.50%
发文量
142
审稿时长
8.3 months
期刊介绍:
Journal is an interdisciplinary publication covering all aspects of structural chemistry, including the theory of molecular structure and chemical bond; the use of physical methods to study the electronic and spatial structure of chemical species; structural features of liquids, solutions, surfaces, supramolecular systems, nano- and solid materials; and the crystal structure of solids.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


