在苯甲醇的有氧氧化过程中使用 MIL-100(V) 作为异相催化剂实现从苯甲醛到苯甲酸的选择性转换†。
IF 4.4
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
合成了一种以钒为中心的金属有机框架 [MIL-100(V)],作为一种异相催化剂,在苯甲醇(Bz-OH)的有氧氧化过程中,只需改变温度,就能将选择性从几乎定量生成苯甲醛(Bz-CHO)转换为定量生成苯甲酸(Bz-COOH)。Bz-OH 的有氧氧化是在 60-120 °C 的温度范围内使用分子氧或空气进行的。在 80 °C 时,Bz-OH 的定量转化率为 98.1%。当氧化温度设定为 100 ℃ 时,Bz-COOH 的生成率达到 100%,同时还实现了 Bz-OH 的定量转化。在有氧氧化过程中,研究了包括由 Bz-OH 生成 Bz-CHO 和由 Bz-CHO 生成 Bz-COOH 分别作为第一和第二阶段反应的串联反应机制的适用性。为第一和第二阶段反应确定的表观一阶速率常数表明,第一阶段反应比第二阶段反应更快。根据所提出的动力学模型,可以计算出由 Bz-OH 生成 Bz-CHO 和由 Bz-CHO 生成 Bz-COOH 的表观活化能分别为 77.3 和 149.2 kJ mol-1。自由基清除实验证明,有氧氧化过程中存在羟基(-OH)和超氧阴离子(O2˙-)自由基。根据 MIL-100(V) 的晶体结构和自由基清除实验所确定的自由基类型,提出了一种机理。这项研究首次利用通过一锅水热反应合成的过渡金属催化剂,为调整对 Bz-CHO 或 Bz-COOH 的选择性开辟了一条新途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
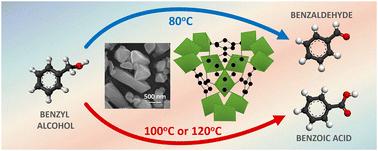
Switching of selectivity from benzaldehyde to benzoic acid using MIL-100(V) as a heterogeneous catalyst in aerobic oxidation of benzyl alcohol†
A vanadium-centered metal organic framework [MIL-100(V)] was synthesized as a heterogeneous catalyst allowing the selectivity to be switched from almost quantitative formation of benzaldehyde (Bz-CHO) to quantitative formation of benzoic acid (Bz-COOH) by changing only the temperature in the aerobic oxidation of benzyl alcohol (Bz-OH). The aerobic oxidation of Bz-OH was performed using molecular oxygen or air in the temperature range of 60–120 °C. A Bz-CHO formation yield of 98.1% was obtained with quantitative Bz-OH conversion at 80 °C. When the oxidation temperature was set to 100 °C, a Bz-COOH formation yield of 100% was achieved with quantitative Bz-OH conversion. The suitability of a serial reaction mechanism including Bz-CHO formation from Bz-OH and Bz-COOH formation from Bz-CHO as the first and second stage reactions, respectively was investigated for the aerobic oxidation process. The apparent first-order rate constants determined for first and second stage reactions demonstrated that the first-stage reaction was faster with respect to the second one. The proposed kinetic model allowed the calculation of apparent activation energies for Bz-CHO formation from Bz-OH and Bz-COOH formation from Bz-CHO as 77.3 and 149.2 kJ mol−1, respectively. The presence of hydroxyl (·OH) and superoxide anion (O2˙−) radicals in the aerobic oxidation was demonstrated by radical scavenging runs. A mechanism was proposed based on the crystalline structure of MIL-100(V) and the radical types identified by the scavenging runs. This study opens a new path for tuning of selectivity towards Bz-CHO or Bz-COOH, for the first time, using a transition metal based catalyst synthesized by a one-pot hydrothermal reaction.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


