通过蛋白质工程拓宽丝氨酸棕榈酰基转移酶的底物范围并将其应用于 3-酮二氢鞘氨醇类似物†。
IF 4.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
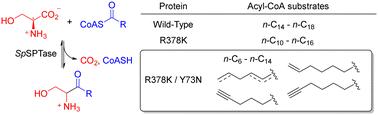
丝氨酸棕榈酰基转移酶通过 L-Ser 和棕榈酰-CoA(n-C16-CoA)之间类似克莱森的缩合/脱羧反应,一步生成 3-酮二氢鞘氨醇(KDS)。遗憾的是,该酶的合成潜力受到其底物范围(n-C14-CoA 至 n-C18-CoA)的高度限制。我们以前曾报道过,Sphingomonas paucimobilis 丝氨酸棕榈酰基转移酶(SpSPTase)的 R378K 变体偏好 n-C12-CoA 等稍短的酰基链长度底物。虽然这是一种改进,但我们仍试图进一步拓宽生物催化剂的底物范围,以便合成范围更广的 KDS 类似物。从 R378K 突变体开始,我们制备了 20 个针对活性位点残基的第二代位点饱和突变体文库。以 L-Ser和n-C8-CoA为底物进行筛选后发现,二十个位置中只有一个位置的突变产生了改良变体(Tyr 73)。酰基-CoA 底物范围以及与 PLP:L-Ser 外部醛二胺的相互作用都发生了显著变化。最好的双突变体(R378K/Y73N)对 n-C8-CoA 具有更高的催化活性(kcat = 0.44 s-1),同时还保留了野生型的热稳定性。它甚至还能接受 n-C6-CoA 和几种功能化酰基链,表明底物范围大大拓宽。最后,为了证明我们的最佳变体的实用性,我们利用 R378K/Y73N 双突变体合成了制备规模的短链 KDS 类似物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Broadening the substrate range of serine palmitoyltransferase by protein engineering and applications to 3-keto-dihydrosphingosine analogs†
Serine palmitoyltransferase produces 3-keto-dihydrosphingosine (KDS) in a single step by a Claisen-like condensation/decarboxylation reaction between l-Ser and palmitoyl-CoA (n-C16-CoA). Unfortunately, the enzyme's synthetic potential is limited by its highly restricted substrate range (n-C14-CoA to n-C18-CoA). We previously reported that the R378K variant of Sphingomonas paucimobilis serine palmitoyltransferase (SpSPTase) preferred slightly shorter acyl chain length substrates such as n-C12-CoA. While this represented an improvement, we sought to broaden the biocatalyst's substrate range further to allow the synthesis of a much wider range of KDS analogs. Starting from the R378K mutant, we prepared twenty second-generation site-saturation mutant libraries targeting residues lining the active site. Screening with l-Ser and n-C8-CoA as substrates revealed that mutations at only one of the twenty positions yielded improved variants (Tyr 73). Both the acyl-CoA substrate range as well as the interactions with the PLP:l-Ser external aldimine were significantly altered. The best double mutant (R378K/Y73N) showed superior catalytic activity for n-C8-CoA (kcat = 0.44 s−1) while also retaining wild-type thermostability. It even accepted n-C6-CoA and several functionalized acyl-chains, demonstrating the substantially broadened substrate range. Finally, to demonstrate the practical utility of our best variant, we used the R378K/Y73N double mutant to synthesize a short-chain KDS analog on a preparative scale.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


