通过苯并咪唑的曼尼希反应合成核苷类似物并确定其特性及其生物活性
IF 0.8
4区 化学
Q4 CHEMISTRY, ORGANIC
引用次数: 0
摘要
新的核苷类似物是由 4-甲氧基苯甲醛和 α-D-甘露糖通过曼尼希反应合成的。芳香醛与邻苯基二胺缩合得到 2-(4-甲氧基苯基)-1H-苯并咪唑,随后与受保护的α-D-甘露糖溴化物发生曼尼希反应,得到新的受保护核苷类似物。后者与甲醇中的甲醇钠发生水解反应,得到目标游离核苷类似物。通过傅立叶变换红外光谱、1H 和 13C NMR 光谱对合成的化合物进行了鉴定,并评估了它们对四种细菌和真菌菌株的体外抗菌活性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
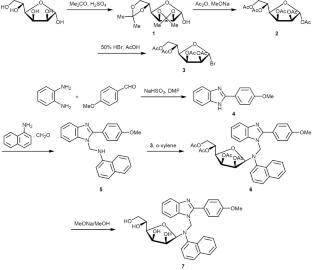
Synthesis and Characterization of Nucleoside Analogues via Mannich Reaction of Benzimidazole and Their Biological Activity
New nucleoside analogues have been synthesized through the Mannich reaction starting from 4-methoxybenzaldehyde and α-D-mannose. The aromatic aldehyde was condensed with o-phenylene diamine to obtain 2-(4-methoxyphenyl)-1H-benzimidazole, and the subsequent Mannich reaction with protected α-D-mannofuranosyl bromide afforded new protected nucleoside analogues. Hydrolysis of the latter with sodium methoxide in methanol gave the target free nucleoside analogues. The synthesized compounds were identified by FT-IR and 1H and 13C NMR spectroscopy, and their in vitro antibacterial activity against four bacterial and fungal strains was evaluated.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
1.40
自引率
25.00%
发文量
139
审稿时长
3-6 weeks
期刊介绍:
Russian Journal of Organic Chemistry is an international peer reviewed journal that covers all aspects of modern organic chemistry including organic synthesis, theoretical organic chemistry, structure and mechanism, and the application of organometallic compounds in organic synthesis.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


