2-Aminothiazole 衍生物的设计、合成、杀菌活性和分子对接研究
IF 0.8
4区 化学
Q4 CHEMISTRY, ORGANIC
引用次数: 0
摘要
为了获得具有更强抗真菌活性的酰胺类化合物,研究人员采用组合化学技术合成了十二种 2-氨基噻唑衍生物。利用 1H NMR、13C NMR、ESI-MS 和元素分析等多种技术对新合成的化合物进行了表征。值得注意的是,化合物 11b 不仅对硬菌有很高的抑制率,而且还具有一定的抗癌作用。分子对接分析为该化合物如何实现其抗真菌效果提供了科学依据。本文章由计算机程序翻译,如有差异,请以英文原文为准。
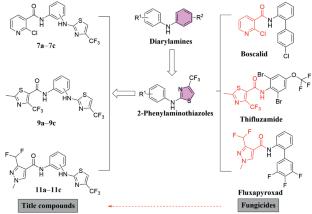
Design, Synthesis, Fungicidal Activity, and Molecular Docking Study of 2-Aminothiazole Derivatives
In order to obtain amide compounds with enhanced antifungal activity, twelve 2-aminothiazole derivatives were synthesized using combinatorial chemistry techniques. The newly synthesized compounds were characterized by using various techniques such as 1H NMR, 13C NMR, ESI-MS, and elemental analysis. Notably, compound 11b not only exhibited a high inhibitory rate against Sclerotinia sclerotiorum but also possessed certain anticancer effects. Molecular docking analysis provided a scientific basis for how this compound achieves its antifungal effects.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
1.40
自引率
25.00%
发文量
139
审稿时长
3-6 weeks
期刊介绍:
Russian Journal of Organic Chemistry is an international peer reviewed journal that covers all aspects of modern organic chemistry including organic synthesis, theoretical organic chemistry, structure and mechanism, and the application of organometallic compounds in organic synthesis.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


